Comment
Disappointing results signal setbacks for Alzheimer’s treatments
Heart failure: charting the clinical trial landscape in the past decade
Russia’s focus on domestic pharma production could shield it from sanctions
Delayed EMA approval: improved safety or lack of access to therapeutics?
CMOs and US Government invest in continuous manufacturing
In Depth
Genomic projects exploit scale as clinical applications play catch-up
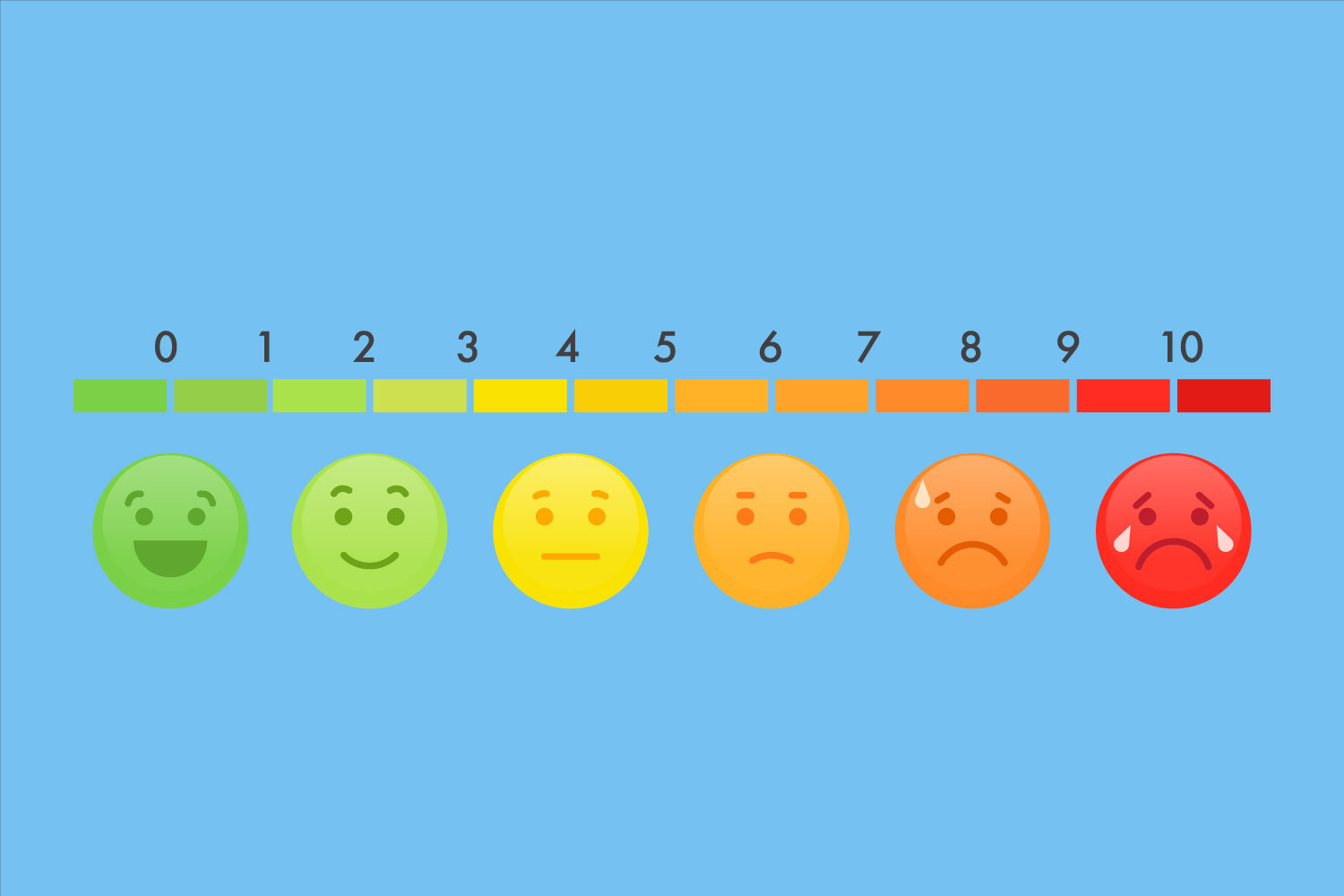
How much does it hurt—measuring pain in clinical trials
Tracing the rise of orphan drug designations over almost 40 years
Neglected tropical diseases: non-profits lead the way as private sectors lags
CMO Moves: Regulatory catalysts for drug manufacturing


Comment
Disappointing results signal setbacks for Alzheimer’s treatments