Company Insight
Sponsored by bioMérieux
Does Your Cellular Therapy Manufacturing Have High Performance Quality Control?
Pharma Technology Focus sits down with Juliana Gutierrez from bioMérieux to discuss how bioMérieux is working with cell and gene therapies and biopharmaceuticals to streamline quality testing to ensure optimal operational efficiency and enhance patient safety.
Main image credit:
Juliana Gutierrez PhD, Scientific Affairs Manager ASPAC - Pharma Quality Control from bioMérieux explains the benefits of SCANRDI® solid phase cytometry system with CELL-BURST®, rapid sterility testing solutions, and BIOFIRE® for rapid mycoplasma testing, explaining the advantages of rapid testing and the innovative technologies powering bioMérieux’s products for cell and gene therapy manufacturing and biopharmaceutical production.
Pharma Technology Focus: According to GlobalData’s Sales and Forecast Database, the cell and gene therapy market is set to surpass $81 billion by 2029, how is bioMérieux working with complex cell and gene therapy projects to provide greater efficiency?
Juliana Gutierrez: The Cell and Gene Therapy market has experienced dramatic growth since 2017, driven by the commercialization of groundbreaking medicines with unprecedented medical value for patients, particularly in haemato-oncological and rare indications. We’re seeing a shift towards earlier lines of treatment and broader disease targets, opening new possibilities to impact more lives positively. While the trend is global, we see particularly high interest in CAR-T cell therapies in China, Korea and Japan where population aging is a critical issue.
This evolution demands advancements in manufacturing processes and technology to deliver quality and safety faster. Innovation is the key to overcome challenges, ensure scalability, and make therapies more accessible and affordable. Without significant improvements in existing process control and product testing technologies, life-changing therapies may remain prohibitively expensive or impossible to produce efficiently.
At bioMérieux, we are proud to be the trusted partner of many cell and gene therapy manufacturers, working together to ensure timely and safer delivery of advanced therapies to critically-ill patients. Our commitment lies in providing innovative testing solutions that help our partners advance quality and safety with assured regulatory compliance and data-driven decision support.
Pharma Technology Focus: What are the testing challenges these therapies have during and after production, and how does bioMérieux assist with overcoming operational issues?
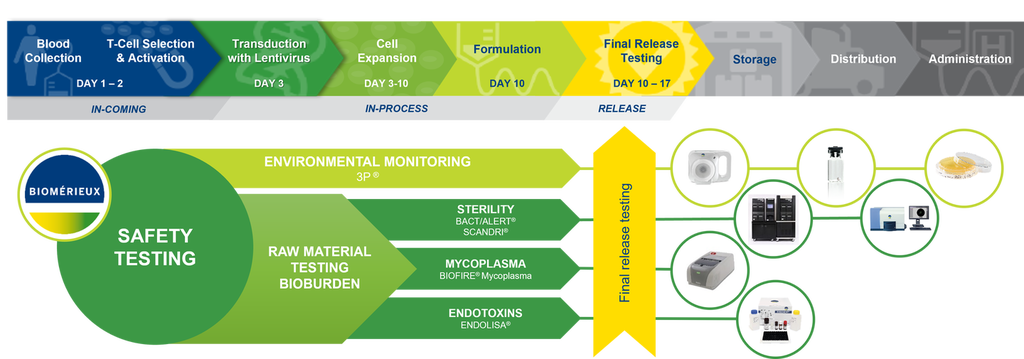
Juliana Gutierrez: Performing quality control (QC) tests for cell and gene therapy products is a complex and often lengthy process, demanding specialized knowledge and facilities, and more time than feasible for these perishable and critically urgent medicines.
Prescribed Compendial sterility tests were designed for terminally-sterilized drug products with sufficient time and product volume for a traditional 14 day test for contamination. This is challenging due to the short shelf-life of cell and gene therapy products, urgent patient needs, and in the case of autologous products, limited material. Regulations are evolving in some countries, but current limitations remain, so cell and gene therapies must often be transfused to patients before sterility tests are completed, potentially putting lives at risk.
The demand for skilled personnel to support QC needs is also challenging due to their shortage. This prompts manufacturers to seek more enclosed, automated, and accurate methods. bioMérieux provides rapid and easy-to-use technologies that enhance manufacturing process efficiencies by streamlining workflows and delivering accurate, actionable results to ensure patient safety.
Pharma Technology Focus: What products does bioMérieux recommend for quality testing in Cell and Gene Therapy manufacturing?
Juliana Gutierrez: Today, many technologies are available for rapid and automated process control and product quality control, enabling significantly reduced analysis times and earlier availability of results. Choosing innovative methods can simplify workflows and enhance efficiency of routine testing. Automation can increase standardization, minimize human errors, and increase data integrity. Implementation of rapid and simple at-line testing of key parameters provides certainty that manufacturing processes are in control. Finally, rapid QC release testing can help get life-saving therapies to patients who need them, quickly and safely.
The innovative BIOFIRE® Mycoplasma solution allows a specialized test traditionally done over 28 days to be completed by anyone, anywhere, anytime in only 1 hour. The BACT/ALERT® 3D DUAL-T simplifies sterility testing of complex products from 14 days to just 5-7 days. The SCANRDI® solid phase cytometry system with CELL-BURST® adapts ultra-rapid sterility testing to the time-critical needs of cell and gene therapy manufacturing. And, ENDOZYME® II GO is the future of bacterial endotoxin testing, using sustainably-produced recombinant Factor C (rFC) for essential safety testing that is more efficient, less prone to human error, and animal-free.
About Dr. Juliana Gutierrez
Dr. Juliana Gutierrez is Scientific Affairs Manager at bioMérieux, where she offers her scientific expertise and support to the bioMérieux Pharma Quality Control business across Asia. With over 7 years of experience in the region, Juliana has held various roles, starting in Japan in Scientific Affairs and more recently in Singapore in Regional Marketing. She holds a PhD in Molecular Biology from the École Normale Supérieure de Lyon, France, and has authored several peer-reviewed papers in biology. Juliana is also a frequent guest speaker at international conferences.
Contact information
David Myatt
Director for Digital Transformation & Communications, Industrial Applications, Asia Pacific
Tel.: +61 401 092 969
Email: david.myatt@biomerieux.com
Website: www.biomerieux.com
