COMPANY INSIGHT
Sponsored by Controlant
Using automation to improve batch release times and product safety
The COVID-19 pandemic illuminated the pharmaceutical industry’s need to rely on technology more than ever as a way to guarantee product safety and availability. Real-time visibility, process automation, and a comprehensive control tower can prevent excursions, reduced time spent on quality issues, and help pinpoint supply chain vulnerabilities.

Automation in the supply chain
Pharmaceutical manufacturers and their logistics partners have specific business rules and policies that they abide by to keep products safe throughout the supply and distribution chain. These can relate to many different aspects of product safety and quality conditions including, temperature escalations, improper location, logistics challenges, pallets being split, light events, suspected theft or tampering, and more. When pharmaceutical manufacturers experience one of these incidents, the entire batch or pallet will have to be investigated and possibly destroyed. Quality assurance (QA) teams spend hundreds of hours every year reviewing and examining quality incidents. Many of those hours could be spent on other functions, like process improvement, if excursion notifications were automated and addressed as they occur.
Real-time supply chain monitoring technology is one tool that QA professionals can utilize to minimize investigation time via alerts about possible incidents as they are happening. Internet of Things (IoT) data loggers and cloud computing are ways to see what is happening to products in real-time. Moving beyond those two tools, having automated processes and workflows can dramatically decrease the time spent reviewing escalations. Automated workflows allow for the immediate release of pharmaceuticals if no excursions have occurred. They also provide the opportunity to prevent excursions with automatic alerts notifying the appropriate parties to look into the issue before affecting product quality conditions.
Control tower visibility
Within the supply chain, multiple stakeholders and hand-off points leave products vulnerable to safety and quality issues. And each party is likely to have its own business system, such as enterprise resource planning, warehouse management, or quality management systems. These platforms all give useful data; however, if they are not automatically linked together into a control tower, organizations are likely missing out on key issues throughout the supply chain. This is especially true when using passive data loggers that only give historical information.
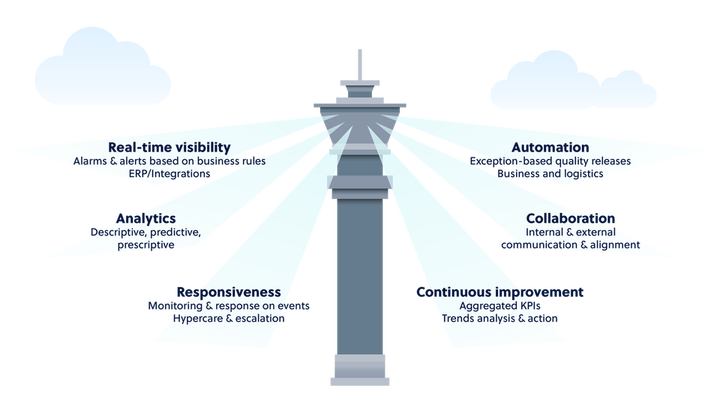
Since the pandemic began, we have seen the pharma distribution model get an overhaul in just a few months, mainly through the integration of multiple business platforms, increased data sharing, and technology-enabled responsiveness, all resulting in an enhanced pharmaceutical control tower. Having an automated and comprehensive control tower means organizations are no longer drowning in data. Parties receive reports that combine information from different systems, people, and organizations into useful data that shows them what is happening, what is likely to happen, and how to prevent issues in the future. Notifications about product quality conditions help stakeholders understand the decisions to be made and what the next steps are so that the shipment is not delayed or damaged. All of this saves time, improves product quality, and safely gets the pharmaceuticals to patients.
Bringing it all together with collaboration
Collaboration has been a hallmark of the pharmaceutical supply chain during the COVID-19 pandemic. Companies that might have formerly considered each other competitors now work side by side to manage and minimize the global crisis. Technology has been a critical source of increased collaboration with all pharmaceutical supply chain stakeholders. Real-time monitoring with predictive analytics builds a cohesive supply chain where parties understand how to make the best decision with the information at hand. Control tower technology is also the most efficient way to automate processes and allow companies to execute planning and strategy in a whole new way. It connects the digital and physical supply chain and links stakeholders across the value chain.
The real-time monitoring technology used in the COVID-19 vaccine distribution empowers supply chain stakeholders to work together and prevent quality issues from happening. At the same time, keeping data over time shows what could happen in the future based on predictive information. An example of this would be seeing that certain products going through a particular airport always have an issue, like going outside approved temperature ranges. Having this information can spur an investigation or offer opportunities for additional training on product handling. Over time, this data will continue to grow, showing more ways to improve processes that prevent waste and stock outages. It can help pharmaceutical companies determine the most effective packaging type or which carriers are best suited for their business model.
When organizations across the supply chain work together and share data, amazing things can happen, which we have all witnessed through the global crisis. The pharmaceutical supply chain has been reinvigorated and made stronger through close collaboration. And technology is fueling the transformation by improving delivery time and product safety through automatic releases, integrating multiple systems into one control tower, and increasing collaboration throughout the end-to-end supply chain.
Contact information
Controlant
Holtasmári 1
Kópavogur 201, Iceland
US Tel: +1 (855) 44-CONTROL
Int’l Tel: +354 517 0630
Email:contact@controlant.com
Website:controlant.com