Device Design & Development

• Patient-centric designs based on human factors studies
• World-class solutions for high volume and high viscosity formulations
• Innovative cartridge-based auto injectors with Needle Isolation Technology (NIT®)
• Advanced technology for next-generation drug formulations
• High-quality and high-volume manufacturing for faster commercialization

• Patient-centric designs based on human factors studies
• World-class solutions for high volume and high viscosity formulations
• Innovative cartridge-based auto injectors with Needle Isolation Technology (NIT®)
• Advanced technology for next-generation drug formulations
• High-quality and high-volume manufacturing for faster commercialization

• Patient-centric designs based on human factors studies
• World-class solutions for high volume and high viscosity formulations
• Innovative cartridge-based auto injectors with Needle Isolation Technology (NIT®)
• Advanced technology for next-generation drug formulations
• High-quality and high-volume manufacturing for faster commercialization

• Patient-centric designs based on human factors studies
• World-class solutions for high volume and high viscosity formulations
• Innovative cartridge-based auto injectors with Needle Isolation Technology (NIT®)
• Advanced technology for next-generation drug formulations
• High-quality and high-volume manufacturing for faster commercialization
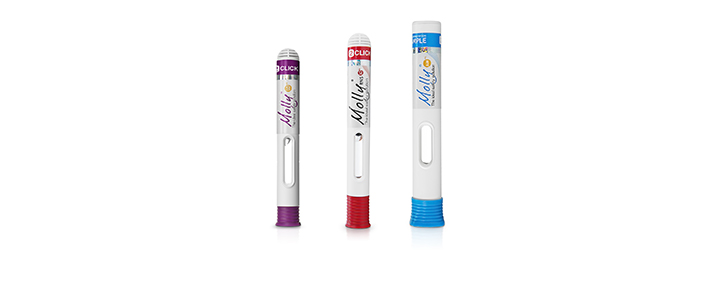
• Patient-centric designs based on human factors studies
• World-class solutions for high volume and high viscosity formulations
• Innovative cartridge-based auto injectors with Needle Isolation Technology (NIT®)
• Advanced technology for next-generation drug formulations
• High-quality and high-volume manufacturing for faster commercialization
Device Design & Development
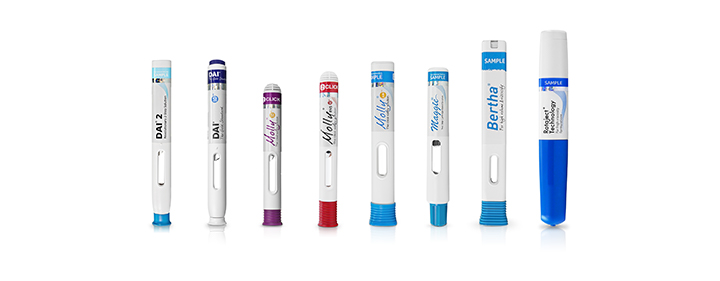
Patient-centric designs based on human factors studies

World-class solutions for high volume and high viscosity formulations

Innovative cartridge-based auto injectors with Needle Isolation Technology (NIT®)

Advanced technology for next-generation drug formulations

High-quality and high-volume manufacturing for faster commercialization
Final Assembly, Labeling & Packaging

Final assembly equipment for cartridge or syringe based drug delivery systems

Total integration between device and machine design and development

Labeling equipment designed for SHL devices

Secondary packaging and/or kitting of assembled, labeled devices

Controlled room temperature (CRT) and cold room storage
Process Development

Analytical Sciences: design, development, validation and transfer of test equipment and test methods, syringe characterization, feasibility testing, transportation and aging studies

Statistics: statistical services assuring optimal control strategy

Project Management: design and planning of tech transfer activities, establishing and tracking of milestones and direct partnering with clients during the entire process
Final Assembly, Labeling & Packaging

• Final assembly equipment for cartridge or syringe based drug delivery systems
• Total integration between device and machine design and development
• Labeling equipment designed for SHL devices
• Secondary packaging and/or kitting of assembled, labeled devices
• Controlled room temperature (CRT) and cold room storage
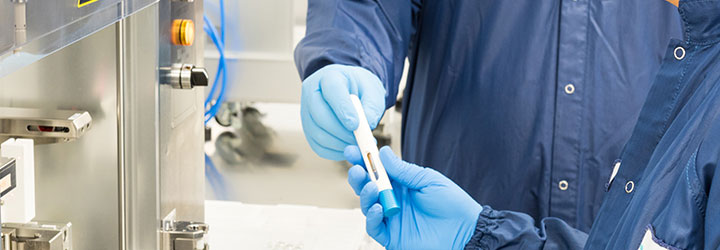
• Final assembly equipment for cartridge or syringe based drug delivery systems
• Total integration between device and machine design and development
• Labeling equipment designed for SHL devices
• Secondary packaging and/or kitting of assembled, labeled devices
• Controlled room temperature (CRT) and cold room storage

• Final assembly equipment for cartridge or syringe based drug delivery systems
• Total integration between device and machine design and development
• Labeling equipment designed for SHL devices
• Secondary packaging and/or kitting of assembled, labeled devices
• Controlled room temperature (CRT) and cold room storage

• Final assembly equipment for cartridge or syringe based drug delivery systems
• Total integration between device and machine design and development
• Labeling equipment designed for SHL devices
• Secondary packaging and/or kitting of assembled, labeled devices
• Controlled room temperature (CRT) and cold room storage

• Final assembly equipment for cartridge or syringe based drug delivery systems
• Total integration between device and machine design and development
• Labeling equipment designed for SHL devices
• Secondary packaging and/or kitting of assembled, labeled devices
• Controlled room temperature (CRT) and cold room storage
Process Development

- Analytical Sciences: design, development, validation and transfer of test equipment and test methods, syringe characterization, feasibility testing, transportation and aging studies
- Statistics: statistical services assuring optimal control strategy
- Project Management: design and planning of tech transfer activities, establishing and tracking of milestones and direct partnering with clients during the entire process

- Analytical Sciences: design, development, validation and transfer of test equipment and test methods, syringe characterization, feasibility testing, transportation and aging studies
- Statistics: statistical services assuring optimal control strategy
- Project Management: design and planning of tech transfer activities, establishing and tracking of milestones and direct partnering with clients during the entire process

- Analytical Sciences: design, development, validation and transfer of test equipment and test methods, syringe characterization, feasibility testing, transportation and aging studies
- Statistics: statistical services assuring optimal control strategy
- Project Management: design and planning of tech transfer activities, establishing and tracking of milestones and direct partnering with clients during the entire process