Your complete clinical trial supply solution, from service to expertise
CLINICAL TRIAL DRUG SOURCING
We leverage long-term partnerships with brand and generic manufacturers to source comparator drugs quickly, manage supply, and adjust for changes.
CLINICAL TRIAL PACKAGING & DISTRIBUTION
We’ve perfected GMP operations to ensure drugs and supplies are stored, packaged, and labeled accurately to be trial-ready at sites around the globe.
CLINICAL TRIAL EQUIPMENT & SUPPLIES
We provide access to and end-to-end supply chain management for key equipment and supplies needed to meet SIV and Patient Visit milestones and achieve consistency across sites globally.
CLINICAL TRIAL PATIENT SOLUTIONS
Our Clinical Trial Prescription Service allows patients to access drugs and supplies via a custom retail and specialty pharmacy network.
Easily Navigate Decentralized Clinical Trials with Myonex
In recent years, the industry has begun to explore decentralized clinical trials (DCT) as a way to reduce rising study costs, increase patient recruitment and retention, and improve patient data by expanding patient pools. The global pandemic has made the DCT concept, which uses telemedicine and home health visits to reduce the need for patients to travel to the study site, even more attractive. However, from protocol development, to getting medications and supplies to patients, to study closure, sponsors can find themselves navigating uncharted waters. Fortunately, innovations in technology, sourcing and other areas are making decentralized clinical trials increasingly feasible.
In the US and Canada, clinical trial prescription services make it possible to ship open-label medications direct to site, directly to patients or to an expansive network of thousands of local pharmacies for patient pick-up with a pharmacy benefit card specifically for clinical trials.
Clinical Trial Prescription Service
Myonex is now the only clinical trial supply company to offer a clinical trial prescription service as part of our comprehensive suite of solutions. Our CTRx service allows patients to access drugs and supplies through a broad Pharmacy and Specialty Pharmacy Network, at zero cost to them.
Clinical Trial Prescription Service Benefits:

no waste

no forecasting

significant cost savings

specialty drug access
Myonex Clinical Trial Prescription Service Process
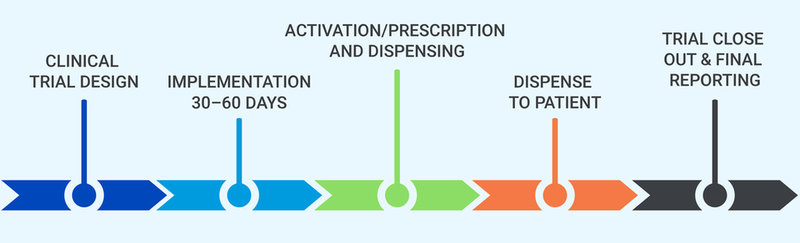
With Myonex as your clinical trial supply partner, you’ll gain…
- expert problem-solving and tailored solutions for any challenge
- speedy access to the right supply of drugs so the trial can start on time
- tools for reducing overage to eliminate waste
- flexibility to change drug supply requests
- ways to reduce costs
- answers to your inquiries
Experience the simplicity of working with a single vendor who can handle:
- comparator drug sourcing and supply
- packaging and distribution
- returning or redistributing unused drugs
- providing all equipment and supplies needed for the trial, from IVs and tubing to refrigeration — and everything in between.
Let’s discuss how we can help you plan your next global clinical trial.
