FINE CHEMICALS FOR PHARMACEUTICALS
from R&D to Pilot and Industrial
more information
How we work...
1.

MGRC
- From few mL to 30 L
- Full GMP if needed
2.
PILOT PLANT
- Located at Catalana
- GL and SS reactors from 100 L to 3,000 L
- Full GMP

PILOT PLAN
- Located at Catalana
- GL and SS reactors from 100 L to 3,000 L
- Full GMP
3.

INDUSTRIAL
- Site assignment based on project needs
- Transfer monitored by MGRC team
- Dual site strategy upon market conditions
Skills
Sites of Moehs group
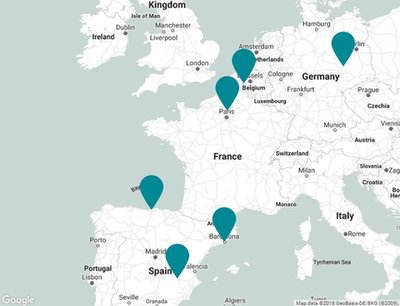
NORCHIM S.A.S.
60340 Saint Leu d'Esserent (France)
BENECHIM S.p.r.l
08191 Rubí (Belgium)
CHEMISCHE FABRIK BERG GmbH
06749 Bitterfeld - oflen (Germany)
MOEHS CANTABRA, S.L.
39313 Polanco (Spain)
PROTEOS BIOTECH, S.L.
02006 Albacete (Spain)
MOEHS IBERICA
Headquarters
08191 Rubí (Spain)
COPRIMA
08213 Polinyà (Spain)
MOEHS CATALANA
08191 Rubí (Spain)
MOEHS BCN
08755 Castellbisbal (Spain)
High quality
standards
Sites of Moehs group
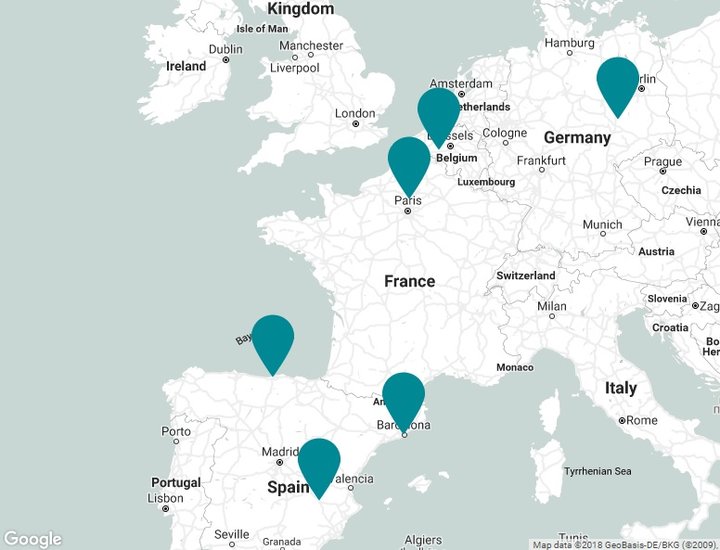
NORCHIM S.A.S.
60340 Saint Leu d'Esserent (France)
BENECHIM S.p.r.l
08191 Rubí
(Belgium)
CHEMISCHE FABRIK BERG GmbH
06749 Bitterfeld - oflen (Germany)
MOEHS CANTABRA, S.L.
39313 Polanco (Spain)
PROTEOS BIOTECH, S.L.
02006 Albacete (Spain)
MOEHS IBERICA
Headquarters
08191 Rubí (Spain)
COPRIMA
08213 Polinyà (Spain)
MOEHS CATALANA
08191 Rubí (Spain)
MOEHS BCN
08755 Castellbisbal (Spain)
Skills

More than 160 reactors running
Capacity up to 1100m3
750 high specialized employees
Fabrication from R&D to pilot, to Industrial scale
High quality standards
Sites approval: FDA, J-PMDA, C-FDA, KFDA, TGA, EMA GMP & ICH Q7A compliance Compliance to USP, Eur. Ph., JP, ND More than 30 CEPS issued
Quality Assurance
Regulatory Affairs
Technical Assistance
Quality Control
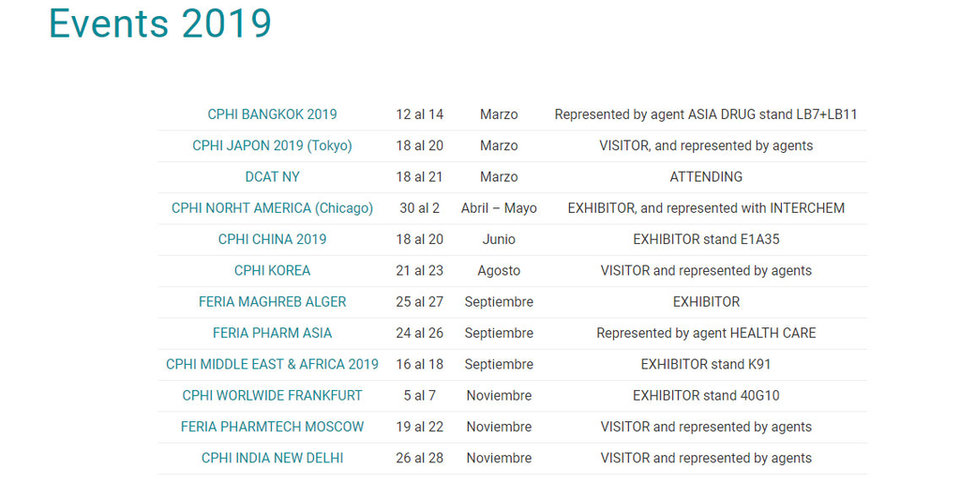
VISIT our Fairs Calendar
Contact us at: