Biodegradable polymers for Injectables and Drug Delivery Devices
PURASORB® polymers are suitable for formulating injectable and implantable drug delivery devices for both veterinary and human use. They are successfully applied in a wide range of controlled release systems for oncology, CNS, pain management as well as many other indications.
Versatility you can rely on
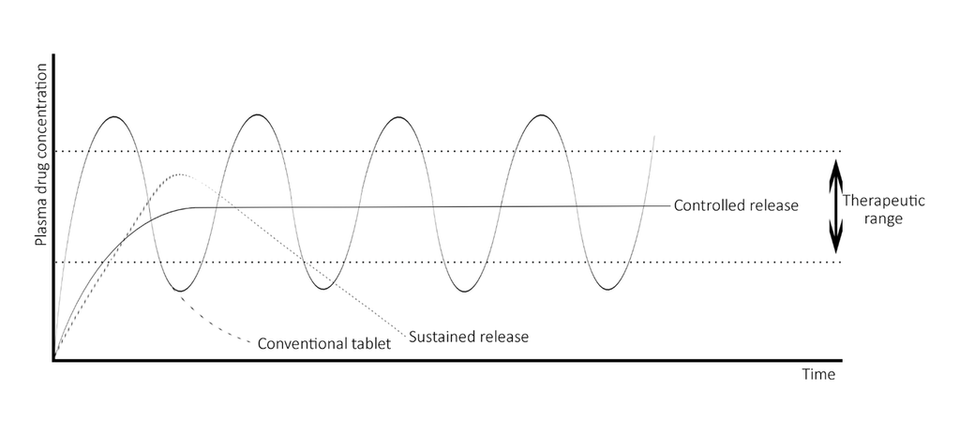
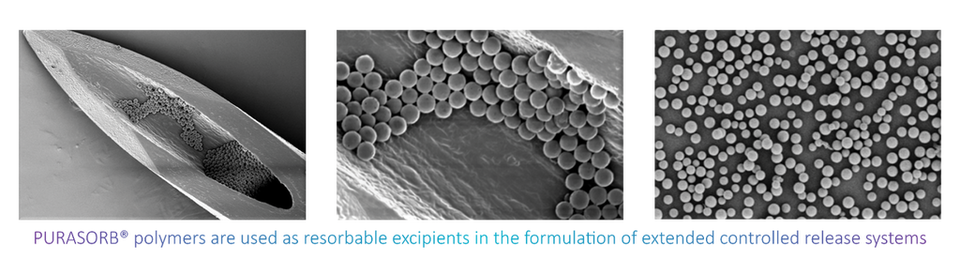
PURASORB polymers are used as resorbable excipients in the formulation of controlled release systems. Using PURASORB in your controlled release formulation enables you to deliver therapeutic levels of a drug to patients over days, weeks, months, with a single injection.polymersomes.
Our polymers can be used to provide a well-established and patient compliant drug delivery solution both for new API’s as well as generic controlled release formulations.
PEG di- and tri-block copolymers
Corbion Biomaterials manufactures high quality polyethylene glycol copolymers. Our DL-lactide / PEG block copolymers (AB and ABA) and DL-lactide/glycolide PEG block copolymers (AB and ABA) are available from small non-GMP laboratory scale to commercial scale in GMP.

Maximum flexibility
Our resorbable polymers allow for maximum flexibility in processing technologies, ranging from extrusion and solvent processing to spray drying.
The properties of our polymers can be tuned to the desired release time and hydrophilicity to facilitate the delivery of a broad range of actives, ranging from small molecules to peptides and proteins.
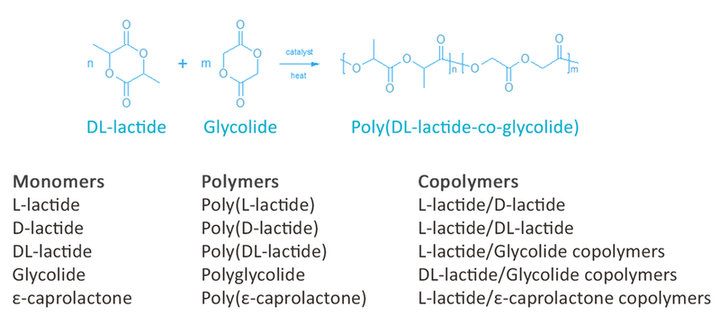
PURASORB resorbable polymers for controlled release systems are commercially available in our PDLG (Poly DL-lactide/glycolide) and PDL (Poly DL-lactides) families. These families of polymers have been used commercially in medical and pharmaceutical applications for many years.
Quality and consistency time after time
Corbion Biomaterials works to the highest quality standards based on ICHQ7 guidelines,
with DMF and MAF files maintained at the FDA. Our commitment is to ensure a high quality, reliable supply of resorbable polymers that meets your requirements now and into the future. We invest continually in our business to guarantee this commitment.
Risk management and dual sourcing
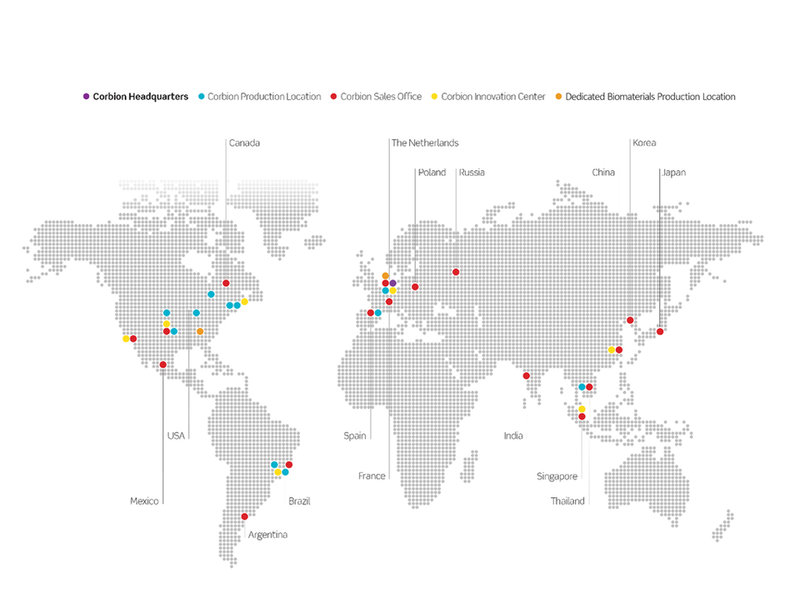
Our dedicated GMP production facility in Europe incorporates proven, large scale technology that enables us to produce a wide range of batch sizes from 5 to over 100 kg with outstanding batch-to-batch consistency. Our GMP production facility in the United States extends and strengthens our manufacturing base even further, bringing the additional reassurance of global supply continuity and reflecting Corbion’s commitment to the Medical Biomaterials domain.