COMPANY INSIGHT
Sponsored by AtoZ-CRO
Your partner in clinical research from A to Z since 1984
As a specialist for studies wordwide, the AtoZ-CRO GmbH offers a broad range of professional services and partner solutions for national and international clinical studies with pharmaceuticals and medical devices
The service comprises the planning and conduction of clinical trials in all indications, patient recruitment and monitoring of the sites. Moreover AtoZ offers pharmacovigilance as well as biometry and statistical services. Medical writing and translation are completing AtoZ’ professional appearance. Customers are small- and mid-sized pharmaceutical, biotech and medical device companies
Due to its close partnership to Eastern Europe, AtoZ-CRO is affiliated with a leading initiator of phase I studies in Warsaw, Poland. The phase I unit is within easy reach for the subjects and in close proximity to a number of large hospitals.
As the AtoZ-CRO performs clinical studies with pharmaceuticals and medical devices, the company is not limited to an indication. Amongst the 300+ performed studies the covered indications range from dermatology, hematology, immunology and internal medicine to neurology, oncology, respiratory disease and several more.
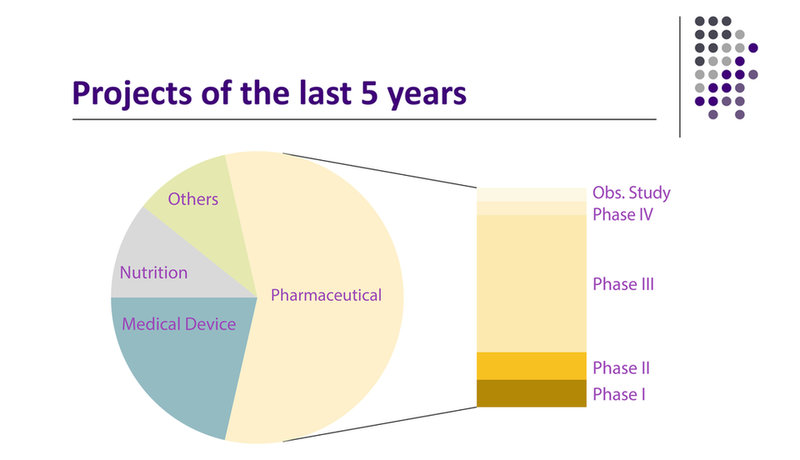
Clinical Studies Phases 0 – IV
From its head office in Overath, Germany, AtoZ-CRO manages and coordinates clinical studies of phase 0 to IV and post-marketing studies for national and international clients all over the world.
As clinical studies will be mandatory for medical devices from 2020 onwards, AtoZ-CRO will be the perfect partner due to its extensive experience not only performing studies in pharmaceuticals but also in medical devices.
The company forms a team of highly experienced professionals and medical specialists ensuring high professional standards in clinical study management. Its services comprise the planning of clinical studies, preparation of study protocols, design and implementation of (e)CRFs and Regulatory Affairs issues.
Short-time patient recruitment
Since its founding in 1984 AtoZ-CRO formed a network of motivated local monitors and investigators all over the world. Close collaborations with leading professional, guarantee very short recruitment periods and highly motivated investigators with experienced staff at study sites.
Moreover studies in Eastern Europe offer the advantage of drug-naïve, highly compliant and motivated subjects and therefore a low drop-out rate. This leads to lower costs per patient and a significant time-reduction in meeting recruitment goals.
Worldwide monitoring
The AtoZ-CRO is not only a specialist in planning but also in conducting clinical studies. Amongst others it offers the recruitment and assessment of study centers, training of investigators and their staff, monitoring the study centers incl. source data check as well as reporting, communication with investigators and the sponsor and supplying the study centers with study supplies and documentation.
Pharmacovigilance
The collating, processing, analyzing and notifying of ADRs during clinical studies is key and one of AtoZ’ competences. The submission of SUSARs and ICSR to European Regulatory Authorities and CECs is done as well as the specialization as an EMA certified safety monitoring for E2B submissions of SUSARs and ICSRs. Medical, clinical and safety regulatory affairs in the EU and a 24-hour physician stand-by service is included as well. Two AtoZ’ employees are certified and empowered to take care of any issue regarding the Pharmacovigilance such as LQPPV.
Biometry and Statistics
To successfully complete a study, AtoZ-CRO offers its Biometry and Statistics services through a long lasting and reliable partner. Amongst others, data collation, validation, analysis and report, including sequential collation and analysis are the main activities.
Quality Management
The company has its own quality management department. Independent audits of study documents (clinical study protocol, subject information sheet/informed consent form, case report form, clinical study report), investigator sites, database and Trial Master Files can be conducted as well as external supplier audits (including special service providers such as IVRS, ECG, clinical trial supply chain management) and internal system audits.
Medical Translation, Medical Writing and much more!
Moreover the AtoZ-CRO offers additional services such as Medical Translation and Medical Writing. Trainings and Seminars (ICH-GCP Basic & Refresher Training for Investigators, Study Nurses and CRAs, Basic Monitoring Training and Communication in Clinical Trials Seminar) can be offered as well.
Company history
Founded in 1984, Dr. Karl-Peter Klein took over as Managing and Medical Director of AtoZ-CRO in February 2007 and led the activities of the company to new dimensions. Based on the company’s long-established international experience in clinical trial management and cooperation with high-rated business partners, AtoZ-CRO is proud to offer a broad range of services covering all clinical research needs, in particular for small and mid-sized pharmaceutical, biotech, and medical device companies, from the planning stages to the final integrated study report.
