COMPANY INSIGHT
Sponsored by Subcuject
Subcuject; a low cost, prefilled wearable bolus injector
Subcuject is developing a prefilled wearable bolus injector, based on osmosis as the driving force. This results in a small, easy to use and low cost device that does not include any electronics for driving or controlling the device.
Given the pipelines with many biologics for subcutaneous administration in larger volumes and with relatively high viscosity, wearable bolus injectors (WBI) have for some years been expected to become a new significant device market. To date, only one product is in reality marketed in a wearable, however, a number of compounds administered using a WBI are now in clinical trials or in registration, meaning that the market will indeed start to grow over a shorter period of time.
The purpose of a wearable bolus injector is to deliver the drug subcutaneously over a longer period of time than what is feasible with an auto-injector or a syringe, whereby the drug is given more time to disseminate in to the subcutaneous tissue. In order to hold a WBI in place over the injection time, it is attached to the skin with an adhesive.
The challenge of delivering higher volumes (>1.5 ml) of injectables over a longer period of time (minutes) is that if e.g. a spring is used, it either requires a loaded spring on stock, which is not desirable, or it requires a user-step to load it, which is also not desirable. Furthermore, it is difficult to slow it down while maintaining enough force to inject a viscous drug and also overcome potential back pressure from the tissue. Therefore, most of the WBIs in development are based on electromechanical solutions based on electronically controlled pumps, whereby a well-controlled slow injection rate can be achieved. The disadvantage of electomechanical WBI solutions are higher cost and, in some cases, assembly of parts by the patient introducing additional user steps.
Auto-injectors have been hugely successful over the later years and especially prefilled, single-use auto injectors. What the prefilled auto-injectors provide is an easy to use, convenient and safe injection solution. Furthermore, auto-injectors have a cost level that does not have significant impact on the total business case for a pharmaceutical company – for most drugs.
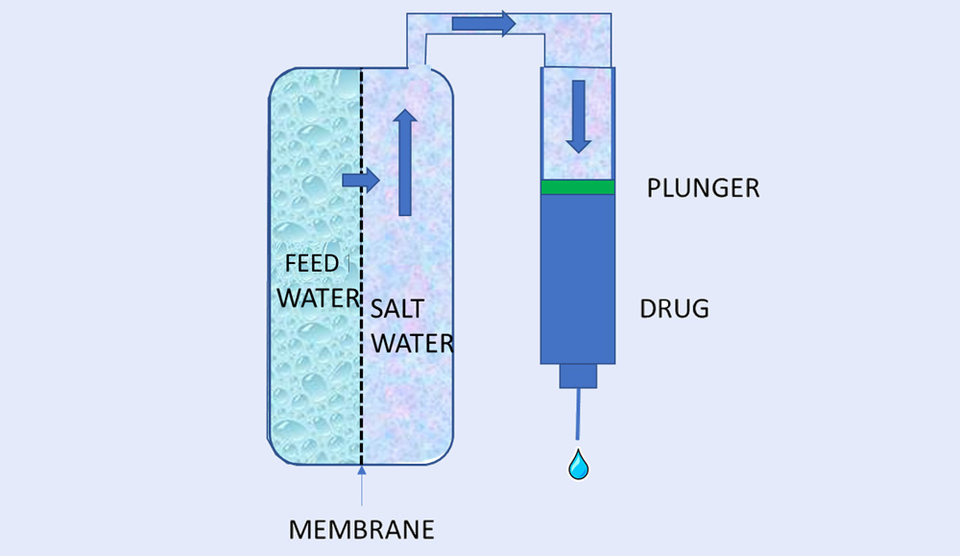
As a response to the challenges of developing a low-cost, prefilled device that is as simple to use for the patient as a prefilled auto-injector, Subcuject is developing a mechanical WBI based on an osmotic power source. Osmosis as a drive force relies on the principle that salt in water tries to reach a concentration equilibrium. Salt is released on one side of a semi-permeable osmotic membrane and as the membrane does not allow salt to pass to the fresh water side, fresh water will be drawn from the fresh water side to the salt side. The excess water is then used to move the plunger in a drug filled cartridge. There is no need for electronics and batteries to drive the device or control the speed as osmosis inherently is a high pressure but low speed drive source..Thereby, a WBI based on osmosis can generate a very high pressure and can, thus, overcome driving high viscosities and driving against high back pressures.
The first implementation of the Subcuject WBI is with a 3 ml cartridge. The device is small, has automatic needle insertion and retraction and injects at a rate about 1 ml/min (for 1 cP in a 30G needle) and the projected manufacturing cost is in line with prefilled auto-injectors. The device is expected to be ready for integrating with the first drug in 2020 for development of the combination product.