Market insight in association witH
Covid-19 Clinical Trials Continue to Rise in Brazil
The number of Covid-19 cases in Brazil has been on a steady increase since the beginning of April, hitting a peak high of confirmed new cases in early August. The number of total cases has surpassed 3.6 million with over 114,000 deaths.
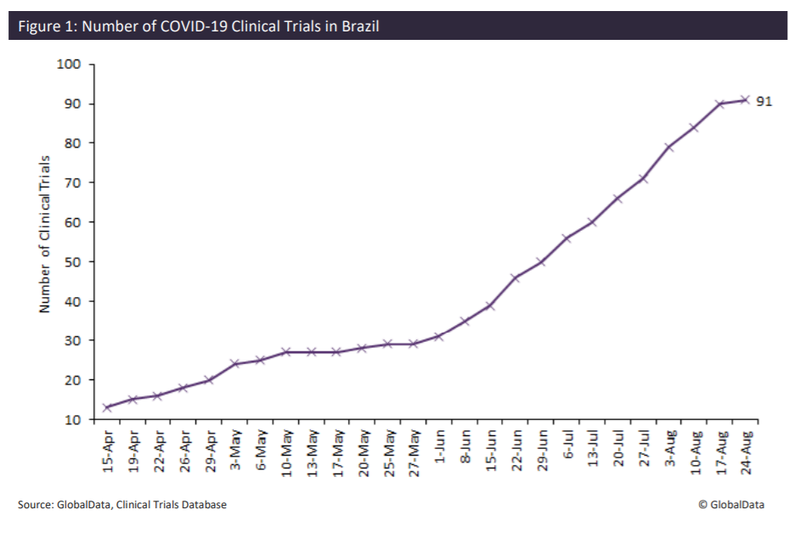
Because of this, the number of Covid-19 clinical trials with a location in Brazil has also been increasing, with a marked steep incline since the beginning of June, as seen in the graph below. There are currently 91 clinical trials with at least one location in Brazil.
The majority are currently ongoing (72%), followed by planned (16.5%), completed (6.6%), and suspended/terminated/withdrawn with 4.4%. Of those trials that have been suspended/terminated/withdrawn, three quarters involved hydroxychloroquine as a primary intervention.
The country’s president, Jair Bolsonaro, is a vocal advocate of hydroxychloroquine and the drug was approved for widespread use across the country by the Health Minister in May for Covid-19. Therefore, it is not surprising that 10.8% of planned and ongoing trials include hydroxychloroquine as a primary intervention.
When looking at clinical trials in Brazil by phase, most are Phase III trials, followed by Phase II. Eight of these clinical trials are in the pivotal/registration stage, the final step before regulatory approval, assuming endpoints are met.
These include two targeted therapies, tocilizumab and lenzilumab, as well as three small molecules, including remdesivir. The other three trials are for vaccines, which are two mRNA vaccines, BNT-162b1 and BNT-162b2, from BioNTech, and an inactivated vaccine, PiCoVacc, from Sinovac Biotech. Brazil recently approved human clinical trials for Ad26.COV2.S, on 18 August, a potential Covid-19 vaccine that is being developed by Johnson & Johnson’s subsidiary Jensen.
The Phase III trial will enrol 60,000 patients, with 6,000 expected from Brazil. Ad26.COV2.S is a prophylactic vaccine that is administered with an intramuscular route of administration.
While the COVID-19 pandemic continues to cause high numbers of confirmed new cases in Brazil, the patient population available for the increasing number of clinical trials should allow these to continue to completion.
For more insight and data, visit GlobalData's Pharmaceuticals Intelligence Centre.