How high resolution FT-IR microscopy is improving tablet formulation across the pharmaceutical industry
CLICK TO LEARN
Analysis of microscopic contamination in pharmaceutical products
Mid-infrared (MIR) microscopy is a useful chemical identification tool that is used to complement traditional light microscopy. Since first coupling an infrared (IR) spectrometer to a microscope, the technique has enjoyed widespread use in a variety of research and industrial applications including the analysis of pharmaceuticals.
In practice, the light microscope is used to locate the region of interest and then it is non-invasively and non-destructively sampled with infrared radiation to provide chemical identification of the desired microscopic area within the sample thus identifying the constituent distribution or any contaminants.
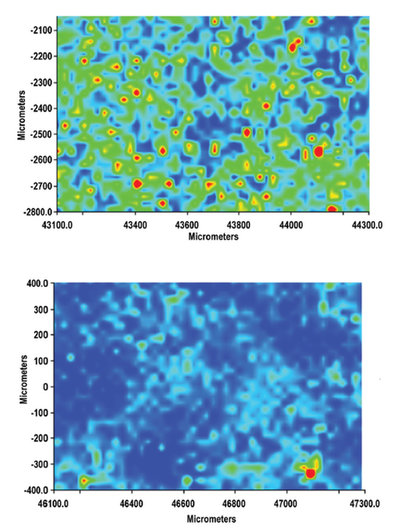
Comparison of scans from a paracetamol tablet with 5% w/w caffeine (top) and a counterfeit paracetamol tablet adulterated with 5% caffeine (bottom). Red areas represent caffeine distribution.
With Innovative Thinking,
We Are Changing Things For The Better
Spectrum 3™

Spectrum 3™ Tablet Checker

Spectrum 3™ Accessories

Spotlight 400 FT-IR Microscope and Spectrum 3™

CLICK TO LEARN
IR microscopy enables the industry to reduce manufacturing cycle times and product variability for shorter time-to-market and with fewer chances of product failures. You can utilize both standard mid-IR attenuated total reflection (ATR) imaging techniques and NIR direct reflectance to gain deeper insights into ingredient distribution in solid doses.
The PerkinElmer Spotlight™ IR microscope systems are designed to meet the challenges of the pharmaceutical industry by generating high-quality, reproducible data from a variety of sample types. The Spotlight 400 FT-IR imaging system combines high sensitivity and rapid imaging with ease-of-use. The ability to image large sample areas rapidly at high spatial resolution extends FT-IR microscopy into new applications. The Spotlight 400 FT-IR imaging system is designed with state-of-the-art technology to allow for intelligent automation and sophisticated analysis. Spotlight and Spectrum FT-IR instruments are 21 CRF 11 compliant and the software and installation services will make meeting regulatory requirements seamless.
CLICK TO LEARN how the Spotlight range of FT-IR Microscopes will help you to deliver better products more quickly.