New Chinese Breakthrough Therapy designation attracts foreign companies
On 31 March, the Centre for Drug Evaluation of China's National Medical Products Administration granted a Breakthrough Therapy designation to Innovent Biologics’ Phase III drug, parsaclisib (IBI376), in follicular lymphoma. Parsaclisib is a small-molecule targeted therapy developed for the treatment of cancer, blood and autoimmune diseases.
This is the most recent review designation awarded by the Chinese regulatory authority, which began offering designations in 2015 to help stimulate pharmaceutical innovation and address unmet needs in China. These designations can offer key benefits to their recipients, including expedited development and review processes.
With this, China joins other existing major markets such as the US, EU and Japan, all of which have had review designations available for more than 20 years. The US first passed the Orphan Drug Act in 1983, allowing for Orphan Drug designations in the country.
China is currently offering Breakthrough Therapy, Accelerated Approval and Priority Review designations.
Priority Review designation directs attention and resources to the evaluation of applications for drugs that, if approved, would be significant improvements over current therapies.
Accelerated Approval designation allows for earlier approval based on a surrogate endpoint for drugs that treat serious conditions and that fill an unmet need, considerably shortening the time required to develop a drug for approval.
Breakthrough Therapy designation helps to accelerate the review of drugs that treat serious conditions and have early clinical evidence to suggest that the drug may demonstrate a substantial improvement over currently available therapies.
Information about China’s Accelerated Approval, Breakthrough Therapy and Priority Review designations can be obtained from GlobalData’s Pharma Intelligence Centre Drugs Database.
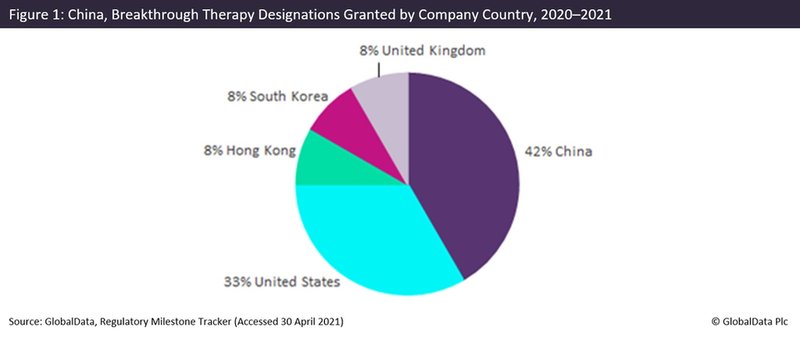
China’s most recent designation is Breakthrough Therapy designation. This was first granted last August to Legend Biotech, a US subsidiary of the Chinese company Genscript Biotech, for its Phase III drug ciltacabtagene autoleucel for refractory multiple myeloma and relapsed multiple myeloma.
Last November, Legend Biotech made headlines for less reputable reasons with the arrest of its CEO Fangliang ‘Frank’ Zhang for the suspected offence of smuggling goods prohibited by import and export regulations.
As shown in Figure 1, the majority of designations have been granted outside China, with 58% of designations having been awarded to foreign companies.
US companies were the main recipients, having been granted 33% of the designations. For example, US-based Amgen was recently granted a designation to its pre-registration drug sotorasib for non-small cell lung cancer.
Despite having been available for less than a year, China’s Breakthrough Therapy designation has proven far better at attracting foreign companies than the previous designations. One example is the Priority Review designation, which has been granted to only 7% of foreign companies despite having been available since 2017.
China has continually sought to make its market more accessible to international drug manufacturers and attract foreign companies by implementing multiple regulatory changes, to allow for faster drug approval timelines and clearer guidelines.
Its adoption of review designation such as Priority Review, Breakthrough Therapy and Accelerated Approval aims to expedite promising new therapies for serious conditions, helping to combat unmet needs in the market.
For pharmaceutical industry data, comment and analysis, visit GlobalData's Pharmaceuticals Intelligence Centre.
Market Insight from