Long HCC pipeline marks change for a previously stagnant market
Despite the high unmet needs within hepatocellular carcinoma (HCC), the market was stagnant from 2007 until 2018, when the US Food and Drug Administration (FDA) approved Eisai’s Lenvima (lenvatinib) in first-line advanced HCC.
Currently, the HCC treatment landscape is continuing to change rapidly. Notably, the combination of Genentech’s Avastin (bevacizumab) and Tecentriq (atezolizumab) for the treatment of first-line advanced HCC, which was approved in May 2020, was more effective than the previous standard of care, Bayer’s Nexavar (sorafenib).
Patients with advanced HCC are not suitable for curative treatments due to the extent of the disease and reduced liver function. Therefore, non-curative treatments such as systemic therapy aim to escalate survival and improve the patient’s quality of life.
Numerous ongoing Phase III clinical trials are aiming to expand HCC treatment options even further. Current mechanisms of action (MOAs) under investigation in Phase III trials to treat patients with advanced HCC include multikinase inhibitors, angiogenesis inhibitors, and immuno-oncology agents (PD-1/PD-L1 and CLTA-4 inhibitors).
Phase I and II pipeline also contains novel MOAs, such as immunomodulators, protein/peptide-based cancer vaccines, a stemness inhibitor, adoptive cellular therapy, and chemotherapy. Key opinion leaders interviewed by GlobalData noted that adoptive cellular therapy is an emerging novel target that may change HCC treatment practice.
The HCC systemic therapy landscape has evolved not only in the advanced stage but also in the earlier stages of HCC. Currently, patients with early stage HCC receive surgical resection, transplantation, and ablation.
While adjuvant therapy has improved outcomes over surgery alone in other cancer types, there are no such therapies to decrease the incidence of recurrence in HCC.
Similarly, patients with intermediate HCC with more widespread liver-confined disease have limited treatment options that consist of locoregional therapies, such as transarterial embolization (TACE).
Systemic therapies are being investigated in combination with surgery as adjuvant therapy for early stage patients and as combinations with TACE for intermediate-stage patients.
Label expansions into early and intermediate HCC would be an effective strategy for companies to gain market share in this space.
Figure 1: Overview of the HCC Development Pipeline.
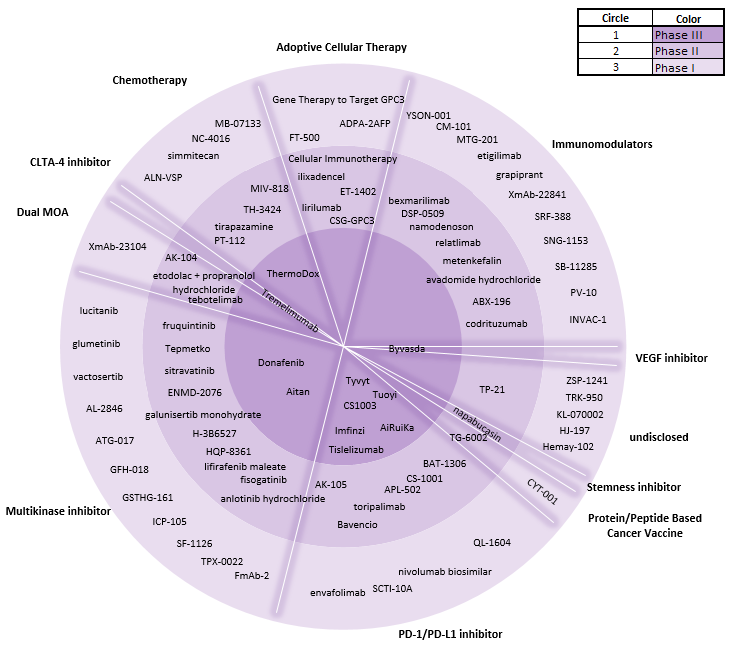
Credit: GlobalData.
The needs in the HCC space are likely to be met, as many new therapies entering the market have different MOAs and are aiming to meet needs in different treatment settings.
The variety of pipeline products for the early, intermediate and advanced HCC settings brings hope for improvements to the cure rate for early stage HCC and survival for all stages.
For pharmaceutical industry data, comment and analysis, visit GlobalData's Pharmaceuticals Intelligence Centre.
Market Insight from