Comment
Emerging microbiome market – unlocking new frontiers
Despite recent approvals, most physicians have no experience with microbiome therapies or use them infrequently. By GlobalData Healthcare.
Credit: Alpha Tauri 3D Graphics v / Shutterstock
The microbiome, which consists of a vast array of microorganisms inhabiting the human body, has gained considerable attention for its role in health and disease.
Recent breakthroughs in microbiome research coupled with the recent approvals of microbiome-based therapies in the US – Ferring Therapeutics’ Rebyota and Seres Therapeutics’ Vowst – are expected to further accelerate the integration of microbiome treatments into clinical settings.
As research into microbiome medicine advances, more therapeutic and commercial opportunities are anticipated to arise, driving the industry forward.
Although at the moment biotechs are leading the charge in pipeline development, larger pharmaceutical companies are expected to become more involved once more successful microbiome therapies are ready to emerge.
Their involvement may take the form of acquisitions, strategic partnerships, licensing agreements, and other collaborative efforts, which will further fuel market growth.
To capture physicians’ opinions and perspectives on microbiome-based therapies, leading data and analytics company GlobalData fielded a survey of 280 physicians from April to August 2024.
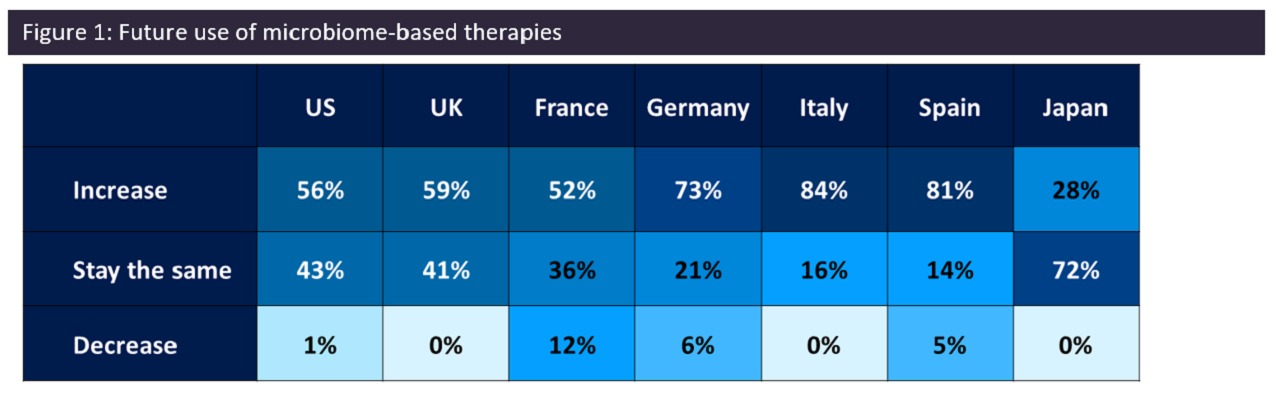
Question: How do you think this is going to change in the next five years? Credit: GlobalData
According to the survey data, the majority of healthcare professionals had either no experience at all with these therapies or used them only infrequently.
However, there was optimism that this situation would change in the next five years, as most surveyed physicians expected the use of microbiome-based therapies to increase during this period – which may be associated with more drugs currently in the late-stage pipeline coming into the market or more research being conducted in the microbiome field.
The exception to this trend is in Japan, where only 28% of healthcare professionals anticipated an increase in the adoption of microbiome therapies in the next five years (Figure 1). This indicates a potential lag in uptake in certain regions.
Initiatives such as educating physicians, developing clear regulatory guidelines, and generating more robust evidence could help to accelerate the awareness of the microbiome.
Currently, infectious diseases and gastrointestinal (GI) disorders are the main focus areas for microbiome research.
This is most likely due to the microbiome’s already proven abilities to improve the function of the GI tract and combat certain infections.
Nevertheless, as research continues to expand the understanding of the microbiome, other therapeutic areas, including oncology, immunology, dermatology, and urology are expected to gain more attention as well.
The potential applications of microbiome-based therapies in these fields present exciting opportunities for future advancements and further diversification of the market.
The survey data also revealed that despite growing interest and optimism surrounding microbiome-based therapies, one of the key barriers to wider adoption remains the lack of sufficient clinical evidence.
The scarcity of robust clinical data can undermine confidence in microbiome-based treatments, making physicians more hesitant to prescribe them over established, evidence-based therapies.
This reiterated once again the need for more clinical research in order to provide the necessary evidence to demonstrate the value of microbiome-based therapies.