

Image: Atomwise CEO and co-founder Abraham Heifets
ESMO 2021: interim analysis backs Keytruda use in first-line cervical cancer therapy
Dr Judith M. Sills. Credit: Arriello
Dr Eric Caugant. Credit: Arriello
At the virtual annual European Society for Medical Oncology (ESMO) congress on 16-21 September, results from the Phase III KEYNOTE-826 study for Merck & Co’s programmed cell death protein (PD) 1 inhibitor, Keytruda (pembrolizumab), were presented, showing that both primary endpoints were met.
Adult patients with persistent, recurrent or metastatic cervical cancer, who were not previously treated with systemic chemotherapy, were randomised 1:1 to receive chemotherapy in combination with either Keytruda or placebo, with progression-free survival (PFS) according to Response Evaluation Criteria in Solid Tumours v1.1 and overall survival (OS) being assessed by investigator review as dual primary endpoints.
Platinum-based chemotherapy in combination with Roche’s Avastin (bevacizumab) and paclitaxel is the current preferred treatment regimen for persistent, recurrent or metastatic cervical cancer, as established by results from the GOG 204 study, in which the median OS was 17.5 months.
In the Phase II KEYNOTE-158 study, Keytruda monotherapy demonstrated a 14.3% overall response rate in patients with programmed death-ligand (PD-L) 1-positive recurrent or metastatic cervical cancer who were previously treated with chemotherapy. The addition of PD-1 inhibition to chemotherapy with or without Avastin in first-line treatment had not yet been examined.
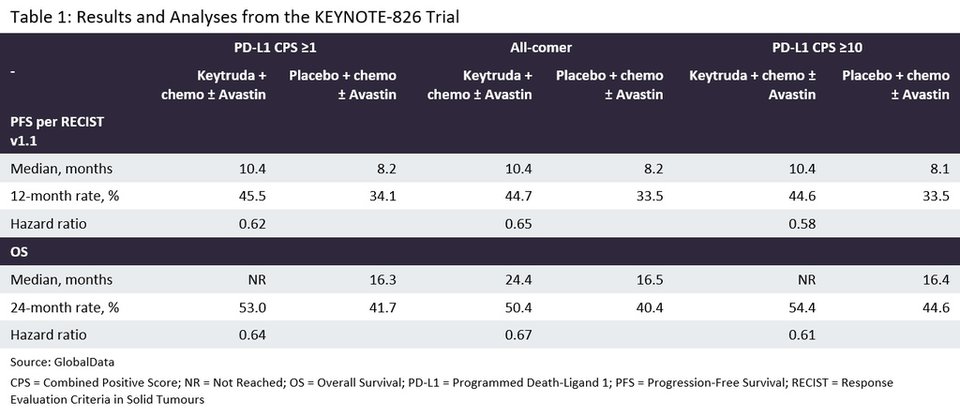
Table 1 indicates that the use of Keytruda and chemotherapy (with or without Avastin) significantly improved both PFS and OS in all three patient populations of the KEYNOTE-826 study.
Grade III or higher treatment-related adverse events were experienced by 81.1% of patients in the Keytruda arm and 75.1% of patients in the placebo arm, but by taking into account that the median durations of treatment were 10.0 and 7.7 months for the respective arms, Keytruda has a satisfactory safety profile.
Having met both dual primary endpoints, the KEYNOTE-826 trial will lead to broader therapeutic options and modify the treatment landscape for patients with persistent, recurrent or metastatic cervical cancer. Merck & Co believes that Keytruda and chemotherapy with or without Avastin can become the new standard of care in this setting.
The company may enjoy a large market share in the first-line segment for this setting, but Merck & Co could have stiff competition from a similar agent in the future.
Sanofi and Regeneron’s Libtayo (cemiplimab) versus chemotherapy significantly improved OS in the second-line treatment of cervical cancer in the Phase III EMPOWER-Cervical I trial, in which Libtayo reduced the risk of death by 31%, with a hazard ratio of 0.69.
Regeneron is expected to file a biologics license application to the US Food and Drug Administration in Q4 2021, but given Keytruda’s preliminary success, Regeneron may also decide to move Libtayo into first-line treatment in the future.
Comment from