Image: Atomwise CEO and co-founder Abraham Heifets
Unmet needs and opportunities for axillary hyperhidrosis
Dr Judith M. Sills. Credit: Arriello
Dr Eric Caugant. Credit: Arriello
The axillary hyperhidrosis (AH) therapeutics market has a considerable unmet need. GlobalData’s upcoming report, ‘Axillary Hyperhidrosis: Opportunity Assessment and Forecast to 2030,’ explores several key unmet needs in this disease space
This includes improved anticholinergic drug delivery systems, therapies causing longer-lasting neuromuscular blockade, increased awareness and education and better insurance coverage for treatments.
Improved anticholinergic delivery systems
Journey Medical Corporation’s Qbrexza (glycopyrronium tosylate), a topical anticholinergic drug, was approved by the US Food and Drug Administration (FDA) in 2018 and has become a game-changer in the AH treatment paradigm.
Before Qbrexza’s approval, antiperspirants were the only choice of therapy. Qbrexza wipes are easy to use with few side effects, resulting in rapid acceptance of the medication by patients and improved adherence. Currently, Brickell Biotech’s Ecclock (sofpironium bromide) is the only anticholinergic pipeline agent in Phase III development.
Despite having superior efficacy to Qbrexza, Ecclock gel does not offer a better delivery method, particularly one that minimizes hand contact with the drug. This is a major opportunity remaining for drug developers in this space.
Longer-lasting neuromuscular blockade
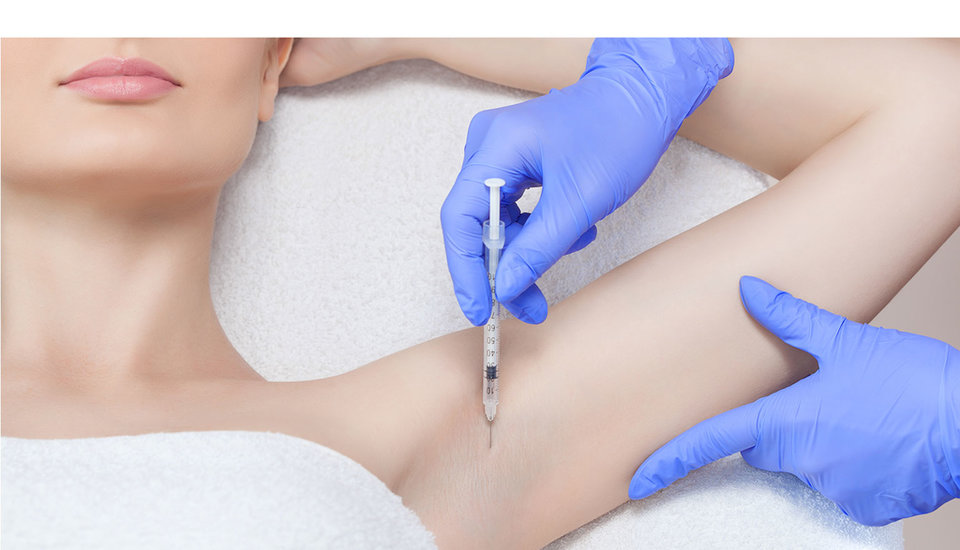
After failing to respond or losing response to antiperspirants and topical anticholinergics, patients with AH are eligible to receive neuromuscular blockade via local botulinum toxin injection.
The only marketed drug in this class that is approved for use in AH is AbbVie’s Botox (onabotulinumtoxin A). However, with only four to six months duration of action (based on the severity of the AH), botulinum toxin therapies present only a temporary solution to symptoms.
Currently, there is an absence of pipeline agents in the neuromuscular blockade drug class. Therefore, the opportunity remains for drug developers to investigate an alternative neurotoxin drug that requires less frequent injection than Botox.
Increased awareness and education
The negative impacts of AH on social functioning are often underestimated. KOLs noted that despite the availability of treatments that can effectively control sweating and improve patients’ quality of life, the majority of patients remain undiagnosed and do not seek treatment.
As such, there remains a significant need for greater investment into educational strategies to increase awareness about AH and the types of treatments that are currently available. This will help to increase the diagnosis rate and hopefully bring significant quality-of-life improvements to the AH patient population.
Improved insurance coverage
Generally, health insurance coverage for AH treatment in the US is limited to patients who meet certain requirements. Regardless of severity, patients must receive prescription antiperspirants as first-line therapy to be able to receive topical anticholinergics as second-line therapy.
In addition, insurance coverage only applies to medications and not to procedures such as microwave energy treatment, miraDry, or surgeries. Therefore, better strategies are needed to improve insurance coverage.
GlobalData believes that the lack of late-stage pipeline products for AH and other unmet needs represent important opportunities for pharmaceutical and biotechnology companies to transform the treatment paradigm for affected patients.
COMMENT from