The SHL Perspective
Empowering Innovation
Maggie® is an easy-to-use, cartridge-based autoinjector built with SHL’s proprietary Needle Isolation Technology (NIT®). Requiring just two simple steps to operate, Maggie® opens up pathways for larger fill volumes and an enhanced injection experience.
Feb 5-6 Paris, France
Booth C64
Learn more at 2020 Pharmapack Europe

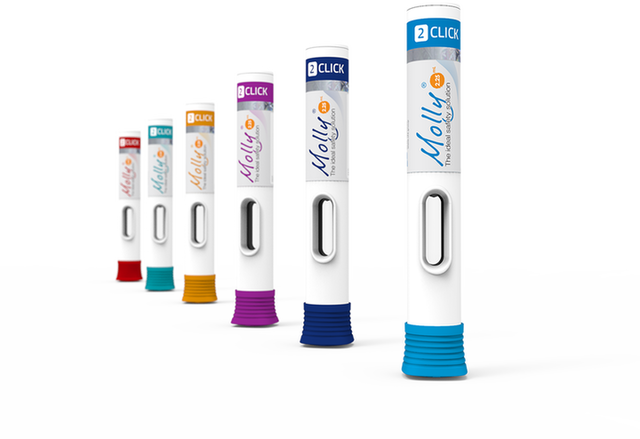
Maggie®
Transform your cartridge-based treatment with Maggie®, a two-step autoinjector built with SHL’s proprietary Needle Isolation Technology (NIT®).
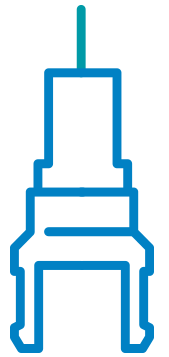
Needle Isolation Technology
Close
NIT® is a unique safety solution that makes it possible for cartridges to function like traditional pre-filled syringes with staked needles. Cartridges support higher volume dosing and enable dual-chamber treatments to be built as easy-to-use autoinjectors.

Higher Volume Injections
Close
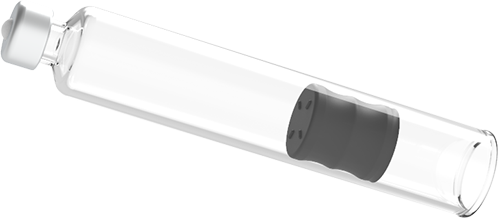
Maggie® is built with a 3mL standard ISO cartridge to support the growing trend for high-volume dosing with the emergence of biologics and biosimilars. Cartridges also offer a level of needle customization for subcutaneous or intramuscular injections.

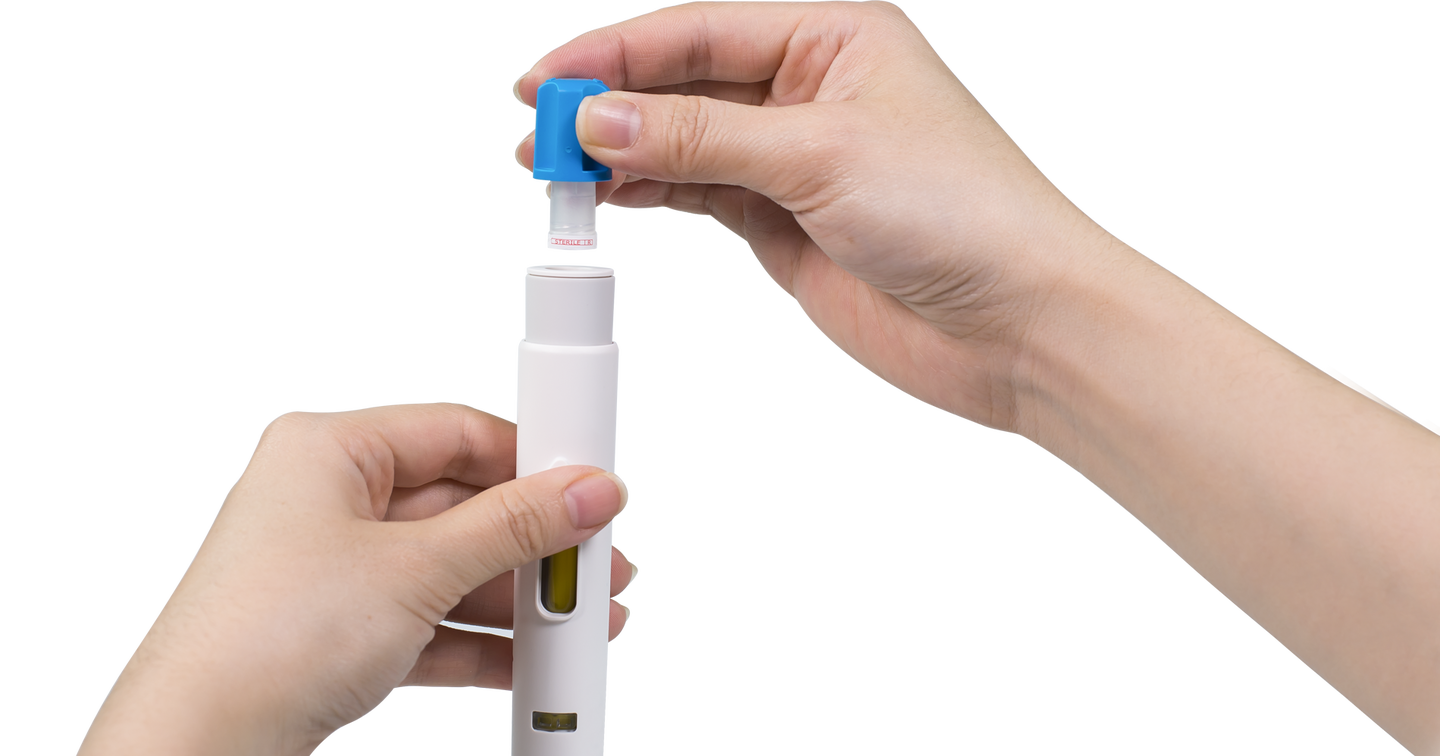
Automated Preparation
Close
Maggie®’s NIT® sub-assembly includes a pre-attached needle. Users simply untwist the cap to attach the needle and open the fluid path. The needle cover automatically extends and the autoinjector automatically primes.


Easy 2-Step Handling
Close
Injection with Maggie® is completed with just two simple steps: uncap and inject. The autoinjector needle cover locks out after injection, shielding patients from being exposed to the needle before and after injection.

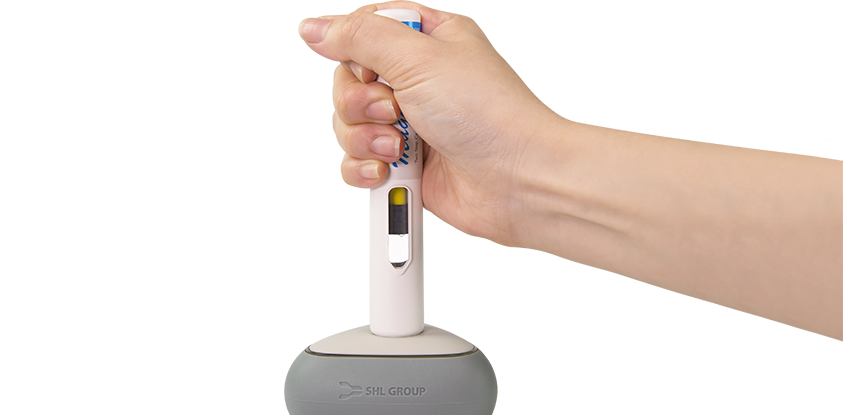
Intuitive Feedback
Close
Maggie®’s large viewing window makes it easier for patients to inspect the drug before and during injection. It also features continuous audible clicks during injection, resulting in an easier determination of dose completion.
Intuitive Feedback
Maggie®’s large viewing window makes it easier for patients to inspect the drug before and during injection. It also features continuous audible clicks during injection, resulting in an easier determination of dose completion.

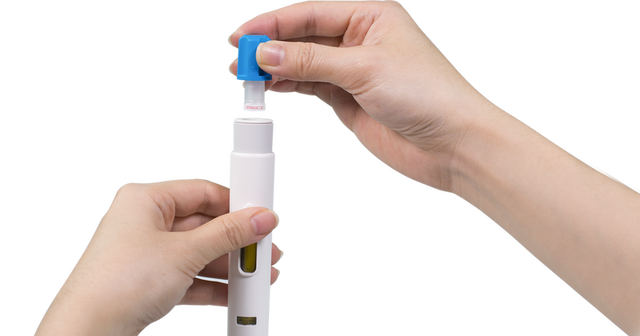
Easy 2-Step Handling
Injection with Maggie® is completed with just two simple steps: uncap and inject. The autoinjector needle cover locks out after injection, shielding patients from being exposed to the needle before and after injection.
Automated Preparation
Maggie®’s NIT® sub-assembly includes a pre-attached needle. Users simply untwist the cap to attach the needle and open the fluid path. The needle cover automatically extends and the autoinjector automatically primes.

Higher Volume Injections
Maggie® is built with a 3mL standard ISO cartridge to support the growing trend for high-volume dosing with the emergence of biologics and biosimilars. Cartridges also offer a level of needle customization for subcutaneous or intramuscular injections.
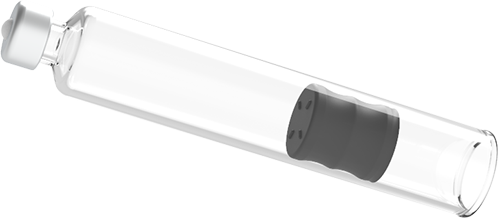
Needle Isolation Technology
NIT® is a unique safety solution that makes it possible for cartridges to function like traditional pre-filled syringes with staked needles. Cartridges support higher volume dosing and enable dual-chamber treatments to be built as easy-to-use autoinjectors.
© 2019 SHL Medical AG. SHL, the SHL logo, and all other trademarks are property of SHL Medical AG and/or its affiliates.