The increasing trend towards self-treatment is driving a dynamic growth in the autoinjector sector. In 2018 alone, end-user sales of drugs in autoinjection systems increased by 31% worldwide.[1] And of the six new drugs for self-injection approved by the US Food and Drug Administration (FDA) during that same year, two were approved in autoinjector formats, with more being developed for future approval.[2][3]
A reason behind the growth of autoinjectors is their ease of use. Autoinjectors are traditionally developed around the pre-filled syringe, which with their staked needles, can be designed into two- or three-step self-injection devices that do not expose the user to the needle. In comparison, cartridge-based injectors require the user to manually attach the needle to the device before injection. This requires an extra step for the user and also poses a risk of contamination and needle stick injuries.
The current growth in biologics and biosimilars has resulted in an increasing need for larger volumes to be delivered through injections. Recent developments in syringe-based systems, which have traditionally been limited to 1mL, have expanded the dosing volume to 2.25mL. In comparison, cartridges can go up to 3mL, with some holding 5mL. For lyophilized formulations that need to be separated from the diluent before use, dual-chamber cartridges offer an additional level of flexibility over their syringe counterparts. Due to this expanded scope of container choices, pharmaceutical companies are increasingly exploring cartridge-based autoinjectors for their drug formulations under development.

Maggie® is a cartridge-based autoinjector that requires just two steps to operate.
An innovative mechanism called Needle Isolation Technology (NIT®) has been developed to address the challenges associated with cartridge-based injection systems. Based on a pre-installed needle hidden inside the device, the technology eliminates the need for users to manually attach the needle. With NIT®, users simply untwist the cap to introduce the needle prior to injection, opening up the fluid path and allowing the injector to automatically prime. Because NIT® makes it possible for the cartridge to behave like a traditional pre-filled syringe with a staked needle, it means that the device can be built into an autoinjector with complete needle covering and shielding before and after injection.
Cartridges also offer a level of needle customization that is typically not possible with pre-filled syringes, including the option to choose the length, gauge, or bevel configuration. And since the needle is introduced only when the device is activated, it also limits the exposure of metal contact with the drug.
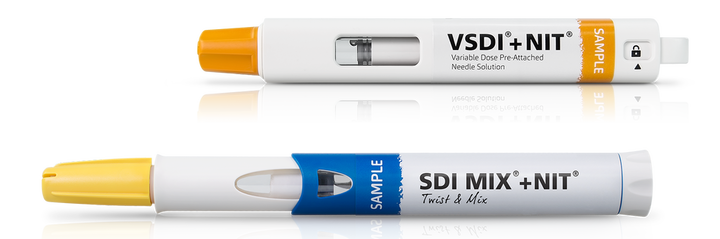
Cartridges offer additional flexibility for variable dosing and dual chamber solutions.the product development timelines.
The first cartridge-based autoinjector built with NIT® was approved by the FDA in 2017 and launched in the US the following year. The device, which is the second-generation device of the pharmaceutical company’s GLP-1 receptor agonist, offers its patients a more convenient injection experience. The autoinjector became the Gold Winner of the 2019 Medical Design Excellence Awards in the Drug-Delivery and Combination Products category.
ABOUT SHL
SHL is a world-leading solution provider in advanced drug delivery systems. We work with leading biotechnology and pharmaceutical companies to develop and manufacture cutting-edge devices, including a range of disposable and reusable injectors with fixed or variable dosing, high dose accuracy and the ability to accommodate different volume/viscosity combinations. These innovative devices can be enhanced through digital implementations to support next-generation healthcare.
References:
- IQVIA. (2018) Retrieved from https://www.iqvia.com/
- Center for Drug Evaluation and Research. (2019). Advancing Health through Innovation: 2018 New Drug Therapy Approvals. US Food and Drug Administration.
- Pharmacircle. (2019). Retrieved from https://www.pharmacircle.com