YES!
WE UNDERSTAND YOU.
EXECUTIVE
EXECUTIVE
Swiss industrial partners strive for high manufacturing quality as well as clinical efficacy in their work. As an innovation partner we collaborate to find solutions, including solving regulatory issues. We build a reliable design and production network to bring the product from idea to realisation.
«Erdmann Design’s active involvement early in the product and service development process is an absolute benefit for Naviswiss. Erdmann creates intelligent medical products and drives innovation.»
Christian Walsoe, CFO, Member of the board, Naviswiss AG, Switzerland
NAVISWISS
New Navigated Surgery
Switzerland
UNDERSTANDING THE DESIGN CONTEXT
What users believe they know about a product strongly impacts how they use it. A mental model is based on belief, not facts.
Designing for others means drawing on knowledge of mental models and requires entering users‘ understanding.
Raimund Erdmann
«I was surprised to find out how similar the design process is to the patient history and documentation which we surgeons use to exchange experiences among each other. Since then we have worked together with designers.»
Prof. Dr. Hans-Florian Zeilhofer, Founder Mininavident AG
DENACAM
3D realtime navigation system for implantology
by Mininavident AG Switzerland
ON THE LEVEL OF USE
The aim of design is to encourage interfaces that move effortlessly from recognition, to exploration, to reliance, until the user is satisfied.
Affordings include direct perceptions: bendability, twistability, pushability, rotatability, graspability, steerability, heaviness, and danger.
Affordances are irreducible cultural habits and difficult to modify. Designers should make them easily recognizable and bring forth the consequences that users expect, ideally without unnecessary constraints.
Raimund Erdmann
MULTISENSORY REDUNDANCY
The design of products and interfaces must assure that all senses are accounted for.
In designing a product, attention to all senses provides the end-user with a richer experience.
Our process includes testing within the context of use. Testing a product in a real-life light and acoustic situation is crucial to the understanding of the user’s behavior and interaction.
Raimund Erdmann
«Humans learn all the time. Meanings change in use. Users should be able to enter the interface of a system on any skill level and advance their competencies at their own pace. Users should also be permitted to introduce variations on successful scenarios, finding shortcuts, economizing efforts, and having fun.
Stryker’s navigation must acknowledge what users know today, but be designed for learnability.»
Project team leader at Stryker
STRYKER
PROFESS Navigation System
Pattern Recognition Optics for Functional
Endoscopic Sinus Surgery
ROBUST SYSTEM
An ideally robust system in one in which most foreseeable mishaps are prevented. When something unintended does slip through, it provides an opportunity for learning something new.
It is a challenge for designers to anticipate what the users of a product might do and provide the necessary clues to prevent interfaces from being disrupted, or worse, causing harm.
Early prototyping with input from medical disciplines and end-users make risks visible, understandable and solvable.
Raimund Erdmann
«Data availability is growing at an exponential rate. Data for patients, for physicians, and for manufacturers. As an industry, we have to get better at using that fragmented data to its full potential by integrating it into new therapeutic concepts to improve outcomes for patients. At the same time, we need to ensure we comply fully with data-privacy law and regulations.»
Cédric Gysel, Human Centered Design Innovation Lab & Make Space Switzerland
JANSSEN
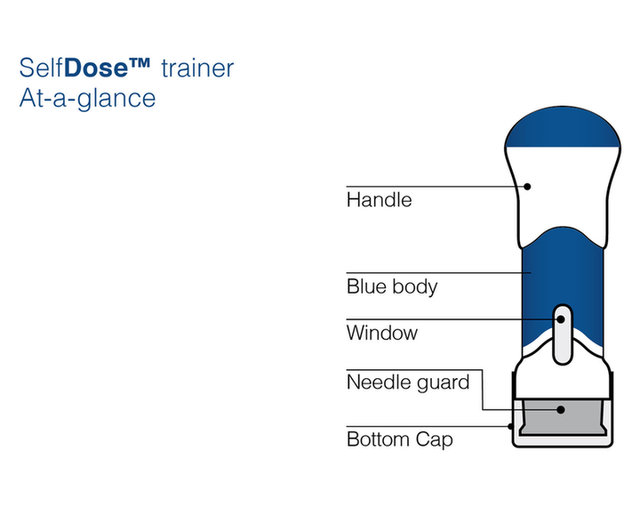
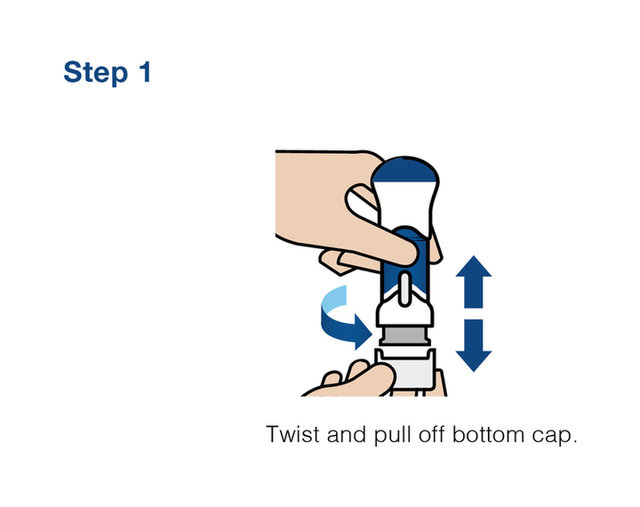
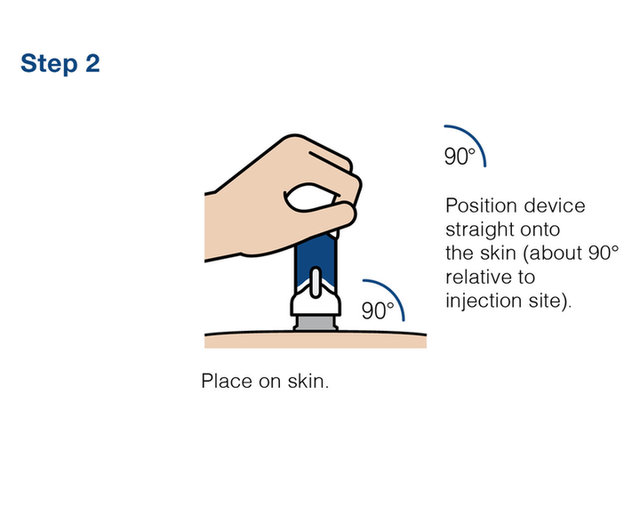
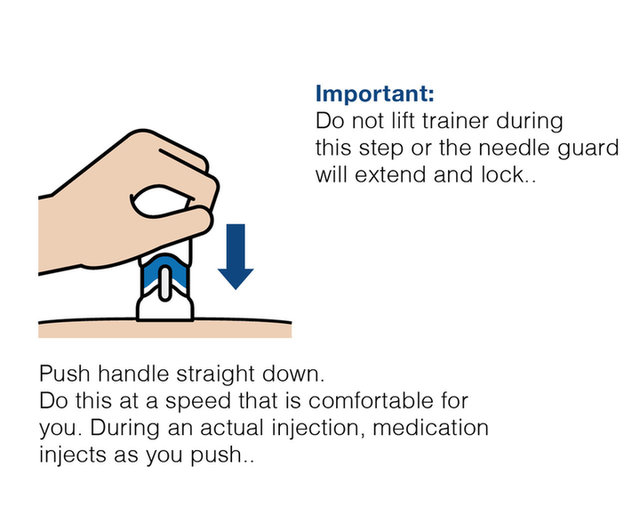
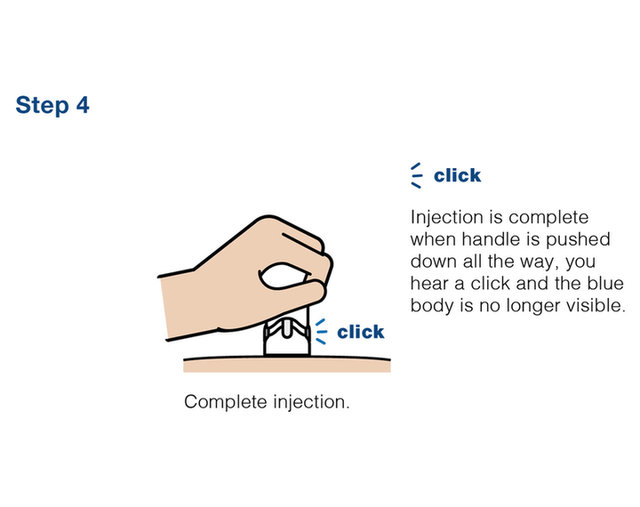
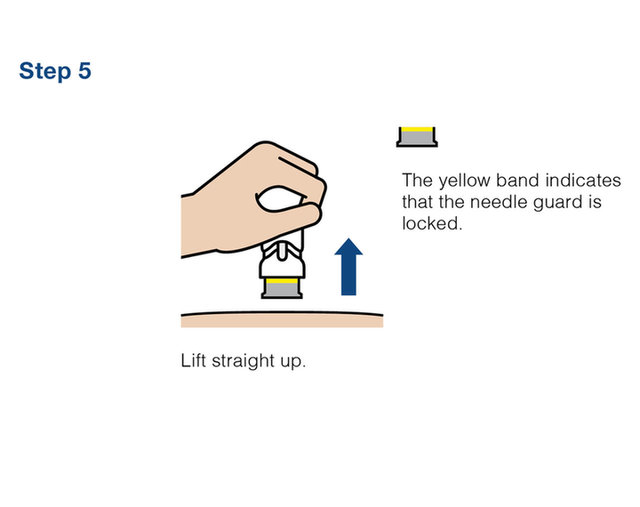
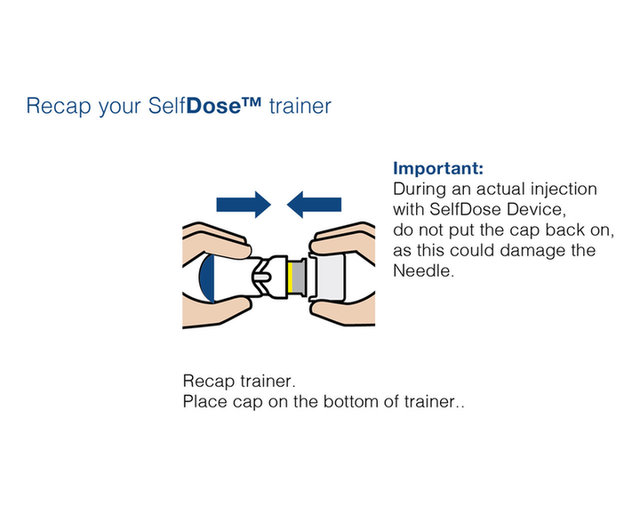
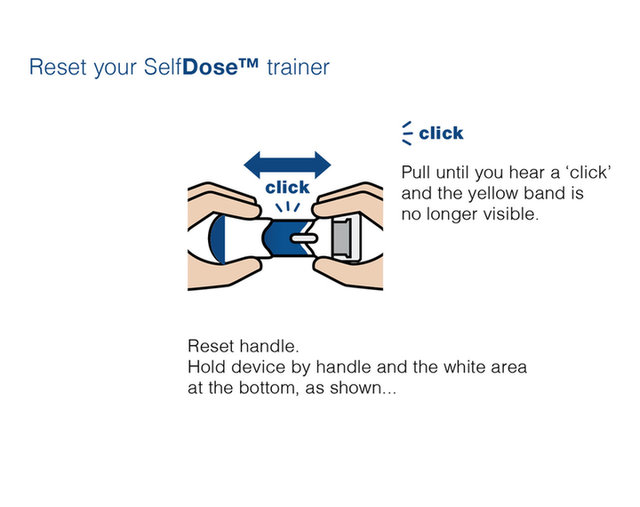
Practice injecting with this reusuable training device. by Cilag, Janssen
Switzerland
INTERACTION FEEDBACK
Feedback on any user action should be as immediate and as direct as possible.
Usability workshops with stakeholders to validate the systems interfaces and interactions will give us the necessary relevance to the task.
Raimund Erdmann