COMPANY INSIGHT
Sponsored by The Solubility Company
The Solubility Company
Standfirst
The Solubility Company Oy is a trusted preclinical contract research organization (CRO). Our mission is to redefine early-stage physicochemical analysis and become the first CRO people turn to for physicochemical measurement in early-stage drug development.
Drug development is a slow and costly process. On average, bringing a new molecular entity (NME) to the market takes 13.5 years and costs USD 0.8B - USD 2.7B. Only 11% of NMEs get through regulatory approval and of those only 30% recover their initial R&D investment. 90% of NMEs in the pipeline have issues with solubility.
At the early stages of drug discovery there are a large number of molecules to be studied, but only small amounts available of each substance. Normally 2-10 mg of material is manufactured when drug discovery projects generate lead compounds in early-stage preclinical drug development. The materials are optimally screened for structure, activity, potency, toxicity, solubility, and morphology.
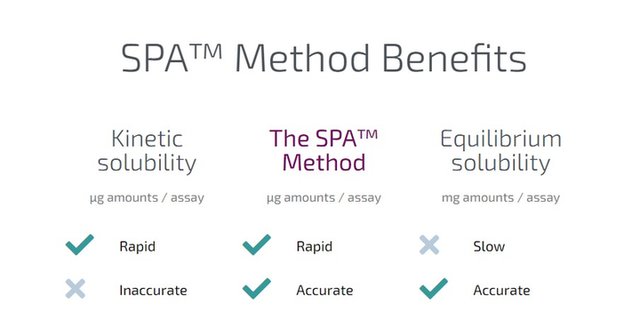
After potency and toxicity, solubility is arguably the most important property of a substance. Solubility is crucial because a substance that does not dissolve will not enter the bloodstream and cannot be effective in the body. Solubility also effects all subsequent decisions regarding developability of the lead compound. Therefore, information about the solubility of the substance is a critical success factor for drug development. Traditional methods to determine the solubility are not used at this early stage as they require large amounts material.
SPA™ Method
Screening of physiochemical properties of the drug candidates is, therefore, challenging and is sacrificed for more urgent in vitro activity and toxicity screening tests. This leads to uninformed development regarding physicochemical properties of the molecules which, in turn, leads to downstream challenges and increased drug development costs.
The SPA™ Method allows the determination of critical but generally delayed information by using only 100 micrograms or less of material. Therefore, the use of the SPA™ Method can provide physiochemical property information for NMEs much earlier in the development pipeline than traditional methods.
The Solubility Company provides measurement services to the global pharmaceutical industry with clients from ranging from start-ups to Big Pharma’s. The services are based on the proprietary disruptive SPA™ technology originally developed at the Division of Pharmaceutical Chemistry and Technology of the University of Helsinki. The SPA™ Method measures the thermodynamic or apparent solubility and related physicochemical parameters of practically any solid sample, with a turnaround of as little as 24h from receipt of sample.
What we offer
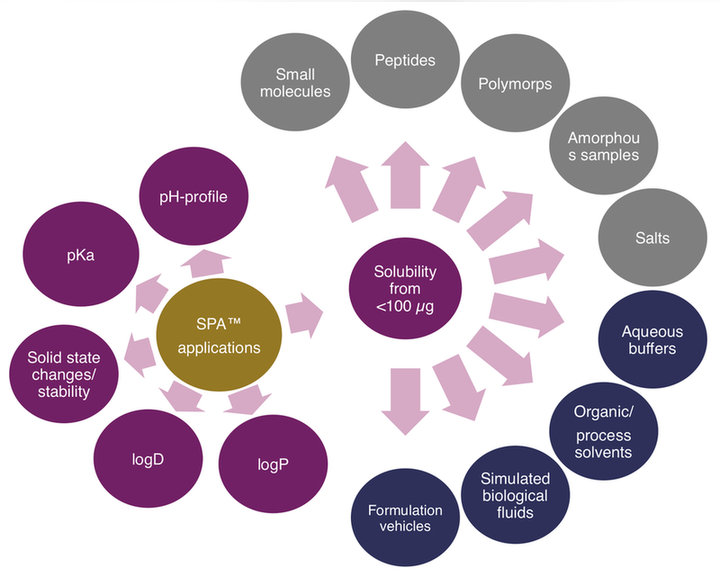
We offer a range of physicochemical measurement services based on the proprietary SPA™ Method requiring less than 100 micrograms per assay:
- thermodynamic/apparent/intrinsic/native solubility in buffer solutions, physiological solutions and organic solvents
- dissolution rate from the SPA™ Method
- possibility for blinded measurement, without requirement of chemical structure information
Higher level physicochemical pKa, logP, logD, pHmax and Ksp can be determined from multiple SPA™ Method readings.
Contact information
The Solubility Company
Viikinkaari 4
00790 Helsinki
FINLAND