The SHL Perspective
Empowering Innovation
SHL's Molly® is an autoinjector designed to address various fill volumes of complex biologics. Built upon a preconfigured technology, Molly® aims to support shortened development timelines for pharmaceutical companies.
Feb 5-6 Paris, France Booth C64
Learn more at 2020 Pharmapack Europe
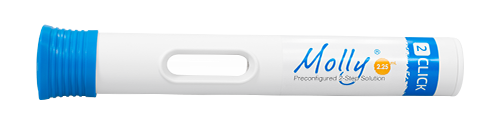
Molly® 2.25 is a large volume variation of the Molly® autoinjector, enabling it to accommodate the high volume requirement of complex biologics. With an upgrade in industrial design that is optimized for production, the new Molly® is designed to meet the evolving requirements of future autoinjectors such as scalable manufacturing.

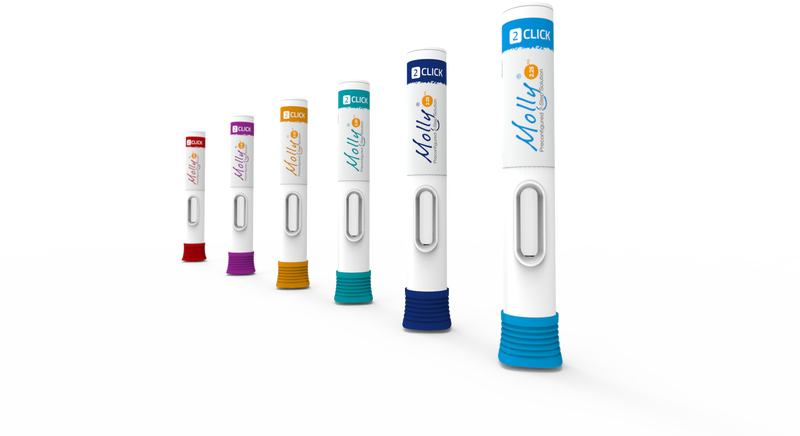
The Molly® autoinjector supports fill volumes in the 1-2.25 mL range, enabling it to accommodate various volume requirements of complex biologics. Molly® is easy-to-use with its two-step operation, and is designed with a flange-shaped cap to prevent unwanted rolling.
Molly® 2.25
Molly® is an incredibly intuitive and easy-to-use autoinjector built with a preconfigured technology developed to minimize customer investments and speed up development timelines.
Bigger Volume
Close
Built for higher volume injections, Molly® 2.25 supports the trends for injection volumes in the 1-2.25mL range. This allows new drugs to be delivered in autoinjector and launched products to be dosed less frequently.
Better Grip
Close
Deepened ridges on Molly®’s cap enhances grip. Its flange-shaped top facilitates cap removal, and its rectangular contour prevents unwanted rolling to ance the device’s safety.
Easier Handling
Close
As a preconfigured offering, Molly® 2.25 is designed to be easily understood and accepted by a variety of patient groups. Having inherited the classic Molly®’s 2-step process, Molly® 2.25 is as compact, intuitive and easy to use.
Faster Timelines
Close
SHL’s final assembly, labeling and packaging services were established to create added value for our partners following the successful design and development of their device, resulting in a fully integrated service from device design to commercialization.
Preconfigured Technology
Close
A high-volume variation of SHL's classic Molly® autoinjector, Molly® 2.25 is a preconfigured device that can also support various levels of customization to meet client specifications.
Preconfigured Technology
A high-volume variation of SHL's classic Molly® autoinjector, Molly® 2.25 is a preconfigured device that can also
support various levels of customization
to meet client specifications.
Easier Handling
As a preconfigured offering, Molly® 2.25 is designed to be easily understood and accepted by a variety of patient groups. Having inherited the classic Molly®’s 2-step process, Molly® 2.25 is as compact, intuitive and easy to use.

Refined Design
Deepened ridges on Molly®’s cap enhances grip. Its flange-shaped top facilitates cap removal, and its rectangular contour prevents unwanted rolling to enhance the device’s safety.

Bigger Volume
Built for higher volume injections, Molly® 2.25 supports the trends for injection volumes in the 1-2.25mL range. This allows new drugs to be delivered in autoinjector and launched products to be dosed less frequently.

Faster Timelines
SHL’s final assembly, labeling and packaging services were established to create added value for our partners following the successful design and development of their device, resulting in a fully integrated service from device design to commercialization.



Molly® 2.25 is a large volume variation of the Molly® autoinjector, enabling it to accommodate the high volume requirement of complex biologics. With an upgrade in industrial design that is optimized for production, the new Molly® is designed to meet the evolving requirements of future autoinjectors such as scalable manufacturing.

The Molly® autoinjector supports fill volumes in the 1-2.25 mL range, enabling it to accommodate various volume requirements of complex biologics. Molly® is easy-to-use with its two-step operation, and is designed with a flange-shaped cap to prevent unwanted rolling.







