Company Insight
Sponsored by Optimal Clinical Trials
A Standout Solution
The Asia-Pacific country and research partner outperforming established markets
Main image credit:
Across the global clinical research landscape, pressure on sponsors and CROs has never been greater. From regulatory holdups to recruitment and retention challenges, cost escalation, and the ongoing push to deliver rapid timelines without compromising data integrity – there are many complexities to navigate.
As a result, sponsors and CROs are increasingly re-evaluating where, and with whom, they run their clinical trials. One country in the Asia-Pacific region and one late-phase research partner in particular, Optimal Clinical Trials, are proving a standout solution.
New Zealand: Asia-Pacific’s proven high performer
A relatively young nation, New Zealand consistently punches above its weight in science, innovation, and engineering.
From being the first to split the atom in 1917 to local aerospace firm Rocket Lab emerging as a leader in rocket launches, New Zealanders are known for being dynamic thinkers, committed to excellence.
In global research, significant medical breakthroughs, including world-first gene-silencing therapies and groundbreaking CRISPR trials, are further evidence of the results New Zealanders are achieving. But it is the country’s mature clinical research ecosystem where this high-performance DNA is gaining the attention of sponsors and CROs.
For global sponsors and CROs, New Zealand offers a combination of attributes rarely found in a single destination:
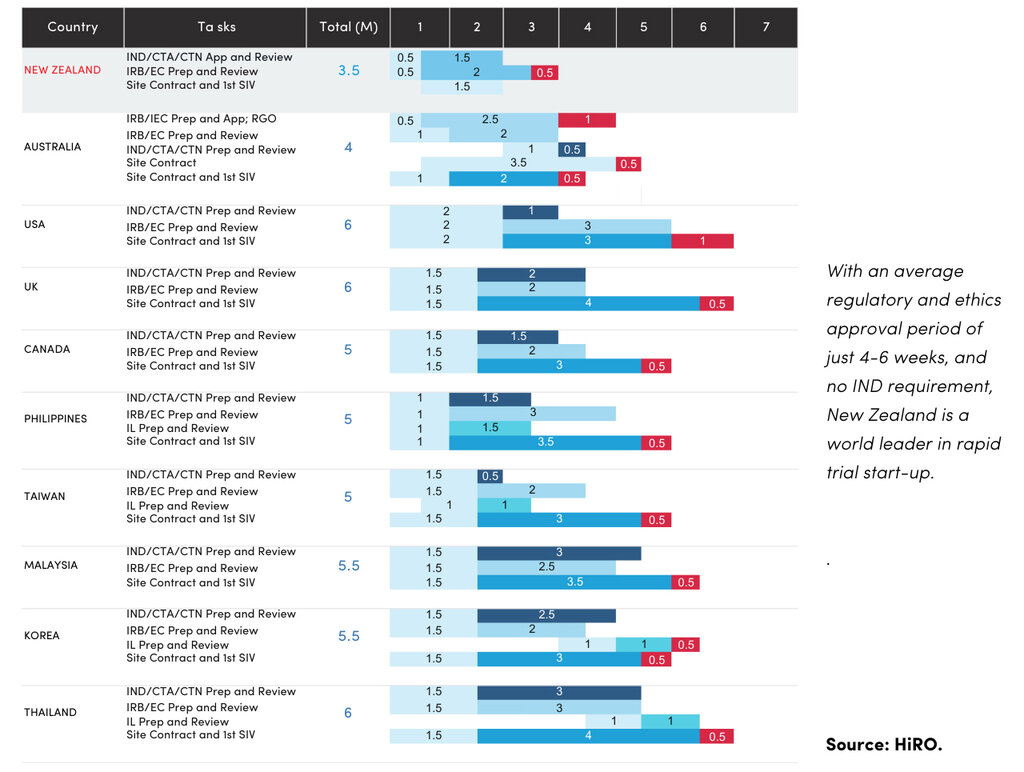
- Fast ethics and regulatory approvals (usually 4-6 weeks), no IND requirement
- Diverse population profile similar to the US and Europe, 72% willing to participate in clinical trials, high vaccine uptake
- Globally recognised skilled workforce, KOLs and experienced PIs
- Mature trial-ready ecosystem and stable economy
- Ability to scale, exceed recruitment targets, and match larger markets
- Cost efficiencies - a favourable exchange rate and 15-43% R&D tax incentives
- Reverse seasonality expands recruitment options
Consider start-up timelines: New Zealand’s streamlined regulatory and ethics pathway enables approvals in just 4–6 weeks - among the fastest globally. With no IND requirement, standardised Clinical Trial Agreements, and a highly coordinated national approach, sponsors and CROs experience faster activation.
New Zealand’s population is also globally relevant and highly engaged – with around 30% of the nation’s population born overseas, it’s considered one of the most diverse countries in the world. Auckland, its’ largest city, is one of the most culturally diverse cities in the world, with 42% of residents born overseas - an invaluable asset for sponsors wanting participant breadth aligned with launch markets.
New Zealanders also demonstrate high willingness to participate in research, reflected in rapid recruitment and some of the world’s highest rates of vaccine uptake.
A frequent contributor to global research, New Zealand’s ability to scale, exceed recruitment targets and match larger markets delivers what many sponsors assume only large, established markets can achieve. In a June 2025 late-phase global flu vaccine trial, New Zealand achieved 112.3% of its target, recruiting the equivalent of 95% of South Africa’s and 86% of Australia’s total enrolment.
And then there is the cost advantage: on top of 15-43% of R&D tax incentive, running a clinical trial in New Zealand can be up to 60% less expensive than the US, 40% cheaper than the UK, and 10% cheaper than Australia, while data quality remains high due to world-class infrastructure and clinical expertise.
Realising the New Zealand advantage: Optimal Clinical Trials
Over the past decade, Optimal Clinical Trials has developed a reputation as a leader in late-phase clinical trials, delivering high-quality results at speed and scale.
Optimal Clinical Trials is also part of the NZCR Group – Australasia’s largest clinical trials network, with complete Phase I-IV capability across New Zealand and Australia.
Unmatched speed & recruitment performance
Optimal Clinical Trials frequently activates sites and achieves first-patient-in within 24–48 hours. Its team’s commitment to speed, quality and results has also earned it the number one spot for highest recruiting site globally on multiple late-phase studies.
Optimal also routinely achieves 95–100% retention - ensuring recruitment success translates into high-quality datasets across 20+ therapeutic areas.
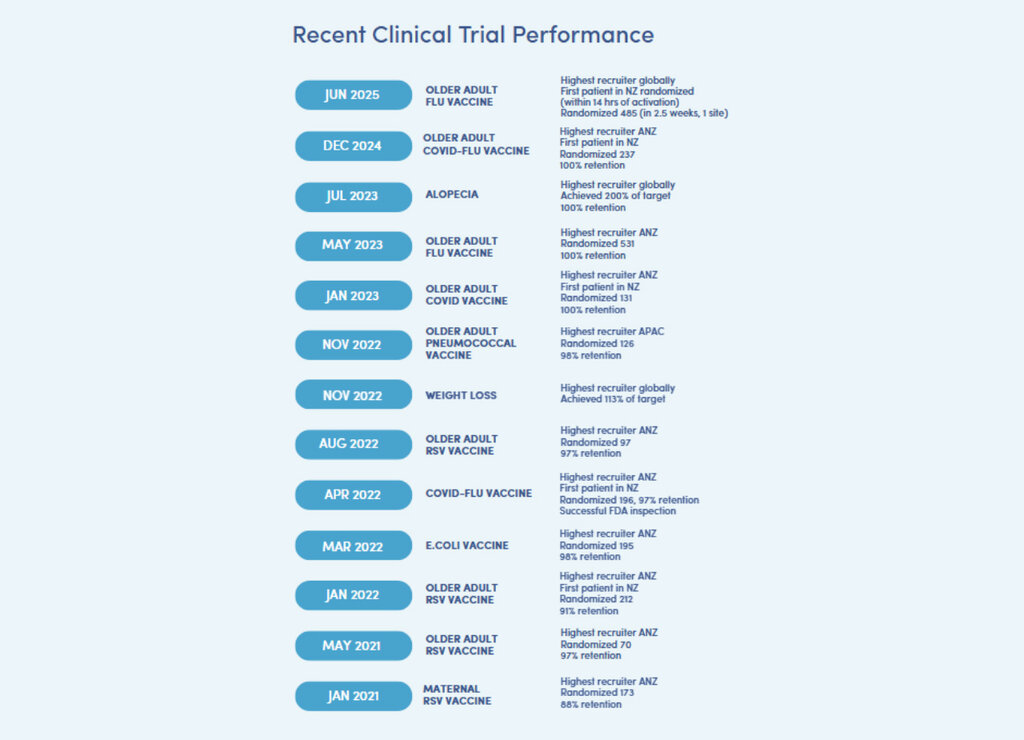
Recent examples include:
- #1 Recruiting site globally out of 45 participating sites for enrolling 485 older adults in 2.5 weeks in a 2025 global flu vaccine trial, enrolled 32% more participants than the highest performing site in the Philippines (a much larger market)
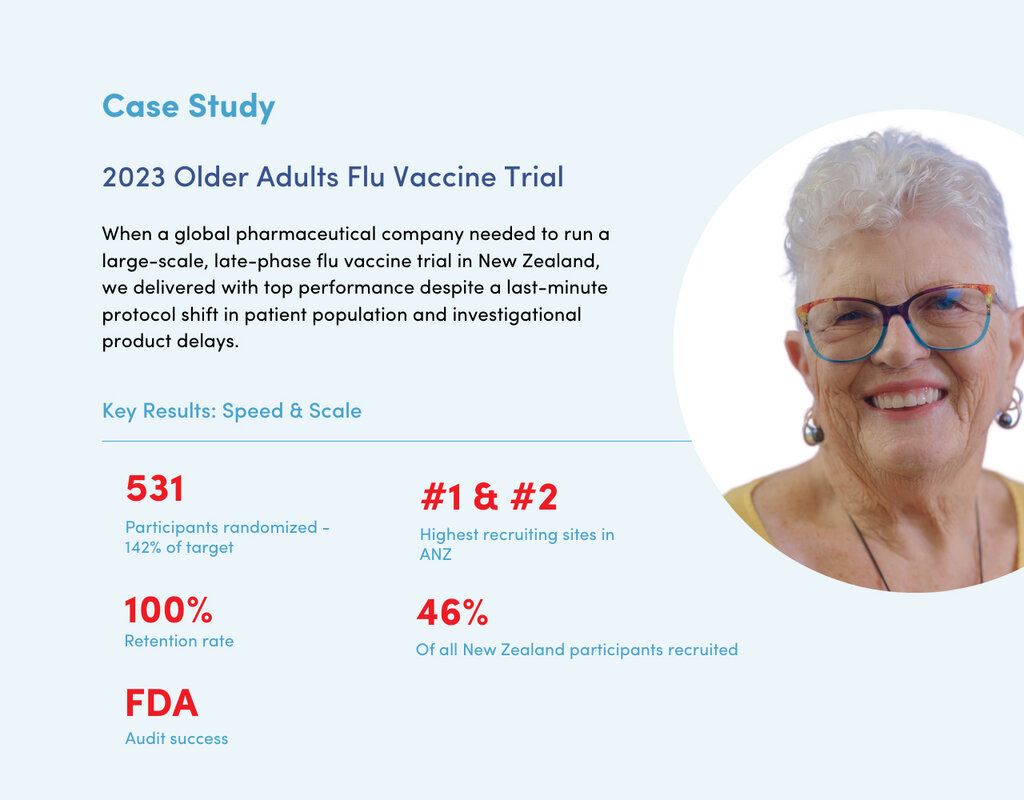
- #1 Recruiting site in Australasia, randomising 531 older adults in a 2023 flu vaccine trial with 100% retention rate
- Fastest and highest global recruiter in a complex obesity trial, contributing strategic data presented at the ADA 2025 conference
- #1 Recruiting site globally, turning around an underperforming alopecia trial as a rescue site in 2023, achieving 200% of its target in just over two months
Access to one of the largest trial-ready populations in the region
Through exclusive partnerships with primary care and pharmacy networks, Optimal Clinical Trials has access to approximately 75% of New Zealand’s primary care patient database. It also has access to a database of more than 153,000 participants (60% are older adults).
World-class quality, accredited systems & regulatory confidence
Optimal Clinical Trials consistently meets the highest expectations data quality. With access to KOLs and PIs, it is ISO 9001 accredited and has a proven track record with FDA audit success. Its team is also recognized for its responsive, collaborative operating culture - reflected in an exceptional Net Promoter Score of 97 from sponsors and CROs in 2024.
Optimal is an accredited Research & Development Tax Incentive Provider.
New Zealand + Optimal Clinical Trials: A future-proof late-phase strategy
For pharma and biotech leaders navigating an increasingly complex global landscape, pairing New Zealand’s regulatory efficiency, diverse and highly engaged population, favourable economics and southern hemisphere recruitment windows with Optimal Clinical Trials’ exceptional delivery record creates a compelling competitive advantage.
If your next late-phase clinical trial requires speed, scale, reliability or rescue-site performance: Choose New Zealand. Choose Optimal.
Contact information
Optimal Clinical Trials
Level 2, 97 Grafton Road
Grafton, Auckland 1010, New Zealand
Tel.: +64 21 538 865
Email: liz@optimalclinicaltrials.com
Web: optimalclinicaltrials.com
