The pharmaceutical, medical devices, and healthcare industries are often risk-averse compared to other sectors when adopting new technologies. However, these sectors have seen an acceleration in digital transformation largely due to the Covid-19 pandemic. IoT-enabled concepts such as mobile health, telemedicine, and RPM give patients greater agency in their health and result in both improved efficiencies and enhanced patient outcomes.
IoT technologies will continue to create multiple opportunities for the pharmaceutical, medical devices, and healthcare sectors, from diagnostics to drug discovery.
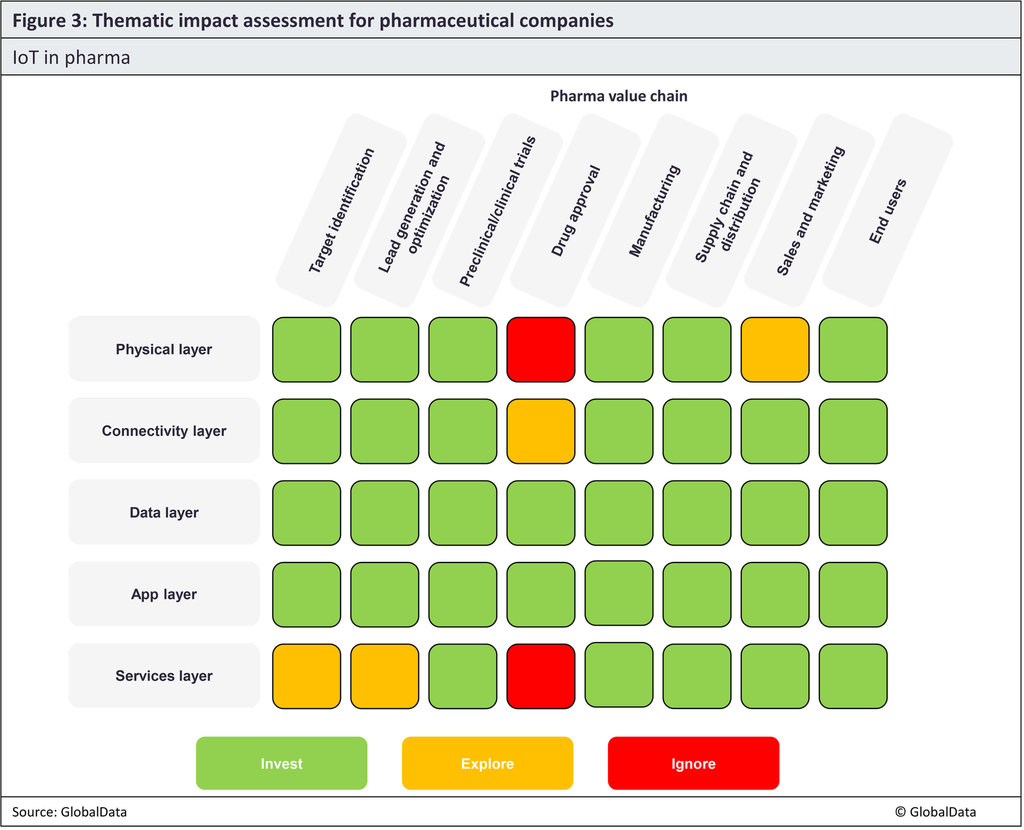
The matrix below details the areas in IoT where pharmaceutical companies should focus their time and resources. We suggest they invest in technologies shaded in green, explore the prospect of investing in technologies shaded in yellow, and ignore areas shaded in red.
IoT tools can be adopted across the entire pharma value chain to various ends. IoT tools are used in the pharma industry, from manufacturing processes to RPM.
For example, industrial monitoring devices control operations within facilities and access real-time status information. Such monitoring devices can alert staff in case of urgent maintenance requirements. Machines such as compressors and conveyor belts can be connected and communicate to reduce waste and energy usage and increase yield and efficiency.
Suppliers of the machines used in manufacturing pharmaceuticals are also trying to make their equipment more user-friendly and easier to debug by using apps and screens at every stage of the process.
The increase in the use and popularity of connected digital devices and health-related mobile apps has produced a novel set of large, diverse, and complex data known as digital biomarkers.
Clinical trials using wearable sensors to monitor or measure patient-derived data will increase, with digital biomarkers being used as primary and secondary endpoints to improve patient outcomes. Ultimately, digital biomarkers will enable better insights into patient health, disease tracking, and preventive medicine, while reducing the cost associated with care delivery.
To increase access to clinical trials, IoT technologies are also used in virtual trials to reduce inconvenience and burden on participants by decreasing the number of physical site visits. Examples of digital technologies used in virtual trials include eConsent, telemedicine, electronic clinical outcome assessment (eCOA), RPM, mHealth, and wearables.
How IoT can help address the challenge of drug discovery and development
Over the past several decades, advances in computational technology have allowed increased exploration of the vast chemical space. CADD and drafting, or in silico approaches (experimentation performed by a computer), are widely used to enhance traditional drug discovery methods and can reduce the time and cost of drug development, with significantly higher hit rates. However, success rates are still low, with just 10% of candidates making it through preclinical development and into clinical trials. The low success rates and the resulting financial cost have led to the need for alternative approaches.
IoT-enabled devices can enhance the R&D process by providing researchers and clinicians with vast amounts of data collected from connected medical devices. The analysis of such large datasets, enabled by artificial intelligence (AI) and machine learning (ML) techniques, will rapidly enhance current drug discovery and development pipelines.
Training AI on the vast amounts of genomic data, health records, medical imaging, and other patient information has allowed an increased understanding of the biological mechanisms of diseases. AI-assisted mapping of disease pathways and protein and drug interactions through specialist vendors has created a deeper understanding of targets and assisted in identifying novel proteins and genes that can be targeted to counteract them.
AI can also be used to predict 3D structures of targets and accelerate the design of appropriate drugs that bind to them through protein structure databases such as DeepMind’s AlphaFold.2. Other AI initiatives include DeepChem and DeeperBind. DeepChem is an open-source software designed for high-throughput screening of small molecule drugs, assessing molecular structure, target binding affinity, and solubility (Qureshi et al., 2023). Alternatively, DeeperBind models the interaction between transcription factors and DNA/RNA by predicting the binding of DNA probes. Such modelling would be particularly useful in understanding the mechanism of action in rare genetic diseases and identifying druggable targets.
While AI has shown the potential to transform drug discovery processes, it faces many challenges. These include the quality and appropriateness of data, continued assurance of drug safety and efficacy, educating the scientific community to increase buy-in, and issues around intellectual property rights. Although the impact of AI on traditional drug discovery is in its early stages, AI-enabled capabilities can substantially speed up and reduce the costs of running expensive experiments when layered into a traditional process, which has the potential to be transformative for patients, medical providers, and the pharma sector.
How IoT can help address the challenge of long and expensive clinical trials
Virtual clinical trials, also commonly referred to as decentralised clinical trials (DCTs), use digital technologies and other processes that differ from traditional trial models to bring research closer to patients’ homes (or other local settings) to increase access to trials. They also reduce inconvenience and burden on participants by decreasing the number of physical site visits.
Making trials more patient-centric is key to improving the overall trial experience and increasing retention rates. A patient-centric study should be designed and executed with participants’ needs at its centre, ensuring that the patient’s voice is incorporated. Engaging patients in study protocol design has been shown to improve research quality, patient outcomes, and relevance to participants, all of which can lead to improved enrolment, adherence, and retention.
Examples of digital technologies used in virtual trials include eConsent, telemedicine, electronic clinical outcome assessment, RPM, mHealth, wearables, and digital biomarkers. The use of virtual trials was increasing steadily before the Covid-19 pandemic, but the pandemic accelerated their use. Since early March 2020, a significant number of companies have announced disruptions to planned and ongoing clinical trials because of Covid-19-related lockdowns and social distancing measures.
The impact on clinical trial participants was the biggest clinical trial-related concern in 2021. Over 1,200 trials have seen disruption due to the Covid-19 pandemic. Due to the large number of disrupted trials, many subjects had their treatment halted or interrupted, or their planned treatment was no longer available. Several trials had slower recruitment due to the impact on sites and investigators to recruit subjects.
How IoT can help address the challenge of supply chain disruption
The pharma and medical device industries are looking for more ways to enhance the productivity of their complex manufacturing and supply chain processes. As technologies advance, improvements in automation technology can lead to substantial enhancements beyond productivity increases. Technologies such as robotics, digital twins, sensors, and AI will allow for fully digitised manufacturing processes.
By disrupting nearly every part of pharma and medical device supply chains, the Covid-19 pandemic highlighted a strong need for improvement and change. The pandemic uncovered inconsistencies in inventory management practices and long-established procurement processes. The pandemic made it more difficult for pharma and medical device companies to manage and improve their supply chains as staff shortages led to disruptions in the manufacturing and supply of raw materials and transportation.
Supply chain disruptions forced companies to favour secure supply chains over efficient ones. The adoption and implementation of technologies such as robotics increased, resulting in better compliance, consistency, and operational excellence. With improved product safety, patient safety can be improved as well.
Companies use digital twins to recreate a digital form of their supply chain to help address constraints and insufficient processes. Digital twins enable manufacturers to finely adjust parameters along the production line through prompt troubleshooting.
The implementation of digital twins can reduce operating costs and extend the lifespan of equipment and assets through applications such as predictive maintenance. The use of robotics in the pharma and medical devices industry is neither new nor uncommon. The convergence of 5G, IoT, and sensors could allow manufacturing robots enhanced with AI to be programmed to continually adjust their performance to achieve optimal productivity and efficiency.
GlobalData, the leading provider of industry intelligence, provided the underlying data, research, and analysis used to produce this article.
GlobalData’s Thematic Intelligence uses proprietary data, research, and analysis to provide a forward-looking perspective on the key themes that will shape the future of the world’s largest industries and the organisations within them.