Market insight in association witH
Trials Delayed Due to Covid-19 Begin to Resume
Since early March, more than 500 companies have publicly announced disruptions to their planned and ongoing clinical trials due to the COVID-19 pandemic. Many companies have delayed the initiation of planned trials or withdrawn the trials completely, and others have suspended enrollment in their ongoing trials or terminated the trials altogether.
The majority of trial disruptions could be attributed to patient safety measures, strict lockdown requirements, social distancing procedures, and the high demand on medical professionals to treat Covid-19 patients.
As the initial peak of the virus is slowly declining and countries are slowly lifting strict lockdown measures, many trials are set to resume activity. On May 28, 2020, GlobalData reported that over 130 trials had resumed activity, with the number of trials getting back on track steadily increasing, and that the US had the highest number of resumed trials. The Covid-19 Dashboard on the Pharma Intelligence Center dynamically tracks both disrupted and resumed trials.
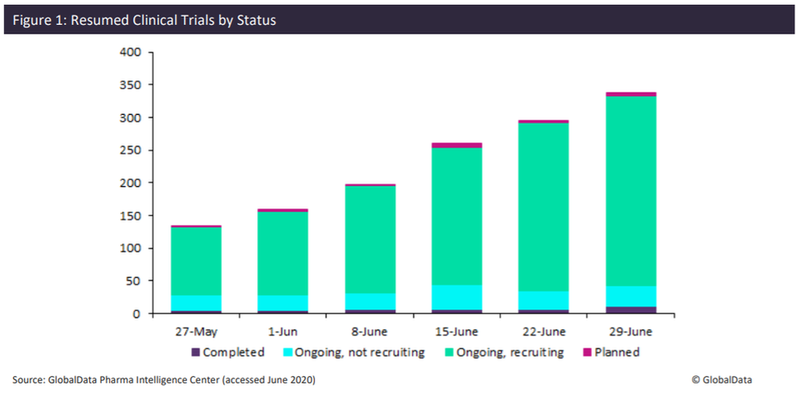
Currently, as of June 29, the number of resumed trials has increased from 130 to over 350: 82.7% of these trials are currently recruiting participants, 9.1% have completed recruitment, but are still ongoing, and 1.1% of trials have yet to start recruiting subjects. The figure shows the steady increase of trials resuming activity.
There has been a gradual increase in the overall percentage of trials for each trial status, the biggest increase being shown in ongoing recruiting and ongoing not recruiting trials. Between 27 May and 29 June, ongoing recruiting trials increased from 67.1% to 82.7% and ongoing not recruiting trials increased from 9.1% to 27.1%. These numbers are expected to rise as lockdown measures globally are eased.
The US remains the country with the highest number of resumed trials at 71.2%, followed by France at 8.0%, the UK at 7.8%, Spain at 7.2%, and Germany at 5.8%. With the US lockdown measures easing and social distancing measures being relaxed, Covid-19 cases are beginning to rise within the US for certain states.
States with a rapid increase include Florida, Nevada, Texas, Arizona, and South Carolina. It remains to be seen if this affects the resumption of trials for companies or sites located in these states and may potentially delay any of the ongoing trials further.
For more insight and data, visit GlobalData's Pharmaceuticals Intelligence Centre.