
Image: Atomwise CEO and co-founder Abraham Heifets
First vaccines, now drugs: FDA’s second wave of emergency Covid-19 approvals
All 12 of the US FDA’s Emergency Use Authorisations (EUAs) last year were for Covid-19-related indications.
Dr Judith M. Sills. Credit: Arriello
Dr Eric Caugant. Credit: Arriello
Last year marked a shift away from US Food and Drug Administration (FDA) Emergency Use Authorisation (EUA) approvals for prophylactic vaccines, as seen in 2020, to EUAs for treatment of serious illness, including Covid-19. The majority of therapies approved in this way were monoclonal antibodies (mAbs), which have high manufacturing outsourcing rates, providing business to numerous contract manufacturing organisations (CMOs).
Even after the approval of several efficacious Covid-19 vaccines, there remained a worldwide unmet medical need that therapies could help to solve. This is because vaccines take time to distribute, their efficacy is not 100%, and certain parts of the population will not receive a vaccine or will not have a strong immune response.
All 12 EUAs in 2021 were granted for Covid-19-related indications (Table 1). Five of these were reissues of EUAs first granted in 2020. Of the remaining seven new EUAs issued last year, just one is a vaccine. Four are monoclonal antibodies, including Genentech USA’s Actemra, AstraZeneca’s Evusheld, Eli Lilly and Co’s etesevimab, and GlaxoSmithKline’s Xevudy (sotrovimab).
Early data suggests that GSK’s small molecule sotrovimab is effective against the Omicron variant of Covid-19, which is currently predominant in the US. mAbs require CDMOs with specialised biologic capabilities, but these services are more widespread than they are for newer modalities such as mRNA vaccines, meaning a greater range of CMOs can benefit from potential manufacturing contracts.
The other two new EUAs are for small molecule oral antiviral pills: Merck & Co’s (Kenilworth, New Jersey) molnupiravir and Pfizer’s (New York, New York) Paxlovid. Merck has outsourced molnupiravir’s manufacture to Thermo Fisher Scientific (Waltham, Massachusetts) and Divi’s Laboratories (Hyderabad, India). Molnupiravir is a potent ribonucleoside analogue that acts as an antiviral. The drug is also approved or has emergency use authorization in the UK, India, Australia, Japan and other countries.
Paxlovid is a combination of nirmatrelvir tablets co-packaged with ritonavir tablets. Data (posted to the bioRxiv preprint server on 19 January) suggest that Paxlovid is effective against the Omicron variant. It is also approved in the UK and European Union (EU), as well as other countries, and is indicated for the treatment of mild-to-moderate Covid-19 in adults and children aged 12 years and older. This gives it an advantage over molnupiravir, which the FDA has approved for adults only.
The US Government has pre-ordered ten million doses of the drug, and Pfizer recently announced that it is increasing its production projections for this year from 50 million to 120 million courses (‘Contract Manufacturing: Opportunities and Threats’ B/POR, November 2021). But this is still far less than the company’s estimate of the global addressable patient population, which it places at 250 million people.
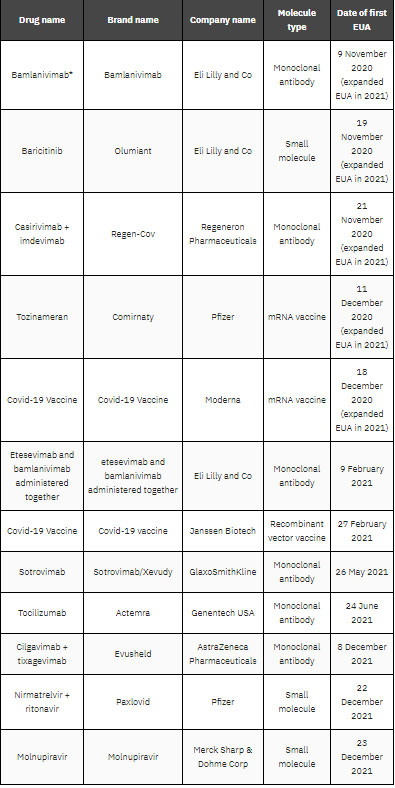
CMO opportunities: all 2021 EUAs outsource production
Figure 1, which is taken from the upcoming GlobalData report New Drug Approvals and Their Contract Manufacture – 2022 Edition, shows that all seven products that received their first EUA last year used CMOs for their manufacture. This is an increase from 2020, when 50% of EUA products had no known manufacturing outsourcing agreements.
Under an EUA, the FDA allows the use of unapproved medical products, or unapproved uses of approved medical products, in a public health emergency if there are no adequate alternatives. The use of multiple EUAs by the FDA is unique to the Covid-19 pandemic.
Comment from