Company Insight
Sponsored by Syngene
Elevating Patient Safety: Syngene’s Leadership in Nitrosamine Testing and Comprehensive Analytical Development Services
Syngene’s Leadership in Nitrosamine Testing and Comprehensive Analytical Development Services
Main image credit:
The pharmaceutical industry has been forced to reckon with the increasing discovery of nitrosamine impurities in drugs, compounds known for their carcinogenic potential. This became especially pronounced in 2018, when several widely used medications, such as Valsartan and Ranitidine, were recalled due to the presence of nitrosamines. Nitrosamines have since become a key focus area for regulatory bodies such as the U.S. FDA, the European Medicines Agency (EMA), and other global authorities. The repercussions of nitrosamine contamination have been costly, not only in terms of product recalls but also in damaging patient trust. These events highlight the critical need for robust, precise, and proactive nitrosamine impurity testing strategies.
The Real-World Impact of Nitrosamine Contamination
The discovery of nitrosamine contaminants has had far-reaching consequences across the pharmaceutical supply chain, directly impacting manufacturers, regulators, healthcare providers, and patients. Since 2018, drug recalls have significantly increased, with over 250 individual drugs withdrawn due to unacceptable levels of nitrosamines.
In 2020, a comprehensive FDA analysis found nitrosamine impurities in over 40% of sampled drug products, leading to global product shortages, delays, and disrupted treatment regimens. Data from the EMA reveal that nitrosamine contamination led to the recall of over 80 million packs of blood pressure medication in Europe alone. This contamination not only posed a significant health risk for patients but also cost the pharmaceutical industry billions of dollars in fines, legal settlements, and lost revenue.
The risk to patients cannot be overstated. Nitrosamines, particularly N-nitrosodimethylamine (NDMA) and N-nitrosodiethylamine (NDEA), are classified as probable human carcinogens. The International Agency for Research on Cancer (IARC) has noted that even low-level, chronic exposure to these compounds may increase cancer risk. This makes stringent testing a critical part of any drug development and manufacturing process.
Table 1: Examples of Major Drug Recalls Due to Nitrosamine Impurities
Year | Drug Class | Active Ingredient | Reason for Recall | Impact |
2018 | Angiotensin II Blockers (ARBs) | Valsartan | Presence of NDMA and NDEA | Global recall, product shortages |
2019 | Antacids | Ranitidine | High levels of NDMA | Global recall, cancer risk concerns |
2021 | Metformin | Extended-Release Form | NDMA contamination | Patient safety, disrupted treatments |
Nitrosamine Impurity Testing Centre of Excellence: A Syngene Differentiator
Syngene offers a state-of-the-art facility for risk assessment, development, and validation of nitrosamine impurities in drug substances and drug products. The facility supports formulation requirements for small molecule APIs, key sourcing materials (KSM), and intermediates. Its internationally accredited analytical labs and highly skilled scientists ensure all the data generated complies with regulatory requirements.
Key features
- Diverse experience in risk assessment, method development, method validation, and testing of nitrosamine impurities in drug products, drug substances, KSM, and intermediates
- All methods developed and validated according to USP General Chapter <1469> in line with current scientific and regulatory approaches. This ensures appropriate control over nitrosamine impurities in APIs and drug formulations
- Availability of skilled workforce, including those with expertise in nitrosamine impurity testing
- Availability of sophisticated nitrosamine analysis instruments such as LC-MS/MS, GC-MS/MS, and HRMS to quantify impurities at the ppb level as per regulatory requirements
- All nitrosamine testing conducted in cGMP labs audited by multiple regulatory agencies
NDSRI risk assessment approach
Syngene has developed a strategic framework to identify at-risk active pharmaceutical ingredients (APIs) for nitrosamine drug substance related impurities (NDSRIs). This framework aligns with recent guidance from the Health Authority and the World Health Organization (WHO) and addresses the industry’s need for clear-cut definitions.
The nitrosamine formation framework is based on the following:
- Understanding the chemistry of the API: In the case of vulnerable APIs like secondary amines, nitrosamines can be formed by reaction with nitrosating agent under susceptible conditions like elevated temperature, acidic conditions, and liquid phase
- Assessing potential mutagenicity/ genotoxicity: Enhanced Ames Test, COMET assay
- Computational toxicology to determine acceptable limits (AI) for NDSRIs: Carcinogenic potency categorization approach (CPCA) reports from Derek Nexus (Lhasa, UK) including acceptable intake (AI) limits
Answering these questions helps determine if an API is susceptible to nitrosation, thereby streamlining compliance with regulatory guidelines.
Comprehensive Analytical Development Capabilities
Beyond nitrosamine testing, Syngene offers a full range of analytical development services, which includes method development, validation, stability studies, and bioanalytical testing. This broad expertise allows Syngene to support clients throughout the entire drug development lifecycle, from preclinical to commercial. Few CDMOs can boast of such a wide range of services, making Syngene a one-stop solution for pharmaceutical companies.
For instance, the company’s stability studies help ensure that a drug product remains safe and effective throughout its shelf life, while its bioanalytical services are critical for the pharmacokinetic profiling required in clinical trials. This integration of services underlines Syngene’s commitment to providing clients with a seamless, end-to-end solution that accelerates time-to-market without compromising on quality or safety.
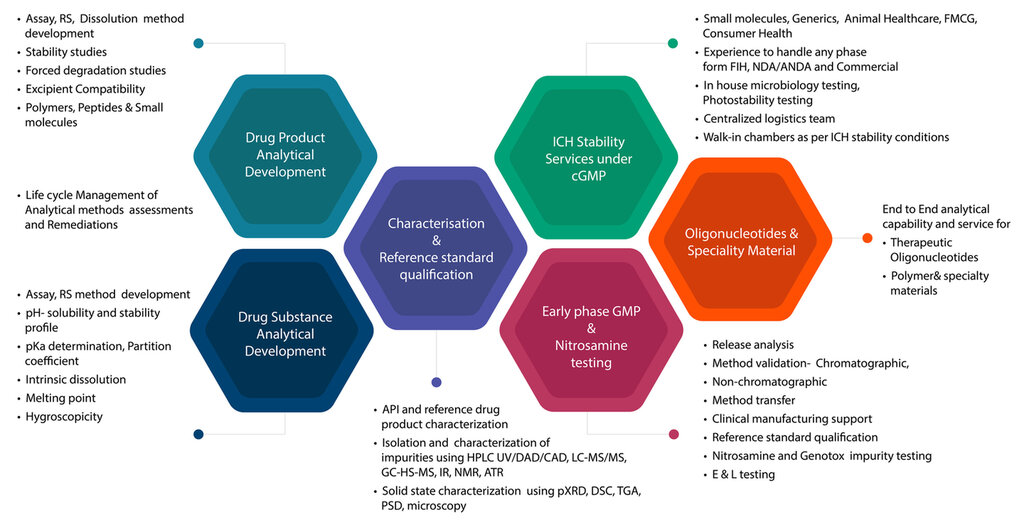
End-to-End Services: A Competitive Advantage
Unlike many of its competitors, Syngene offers a truly end-to-end service portfolio, covering every stage from discovery to development to commercial manufacturing. This seamless integration allows clients to streamline their supply chain, reduce turnaround times, and ensure compliance without having to engage multiple vendors. Syngene’s ability to conduct nitrosamine risk assessments, impurity testing, and even process optimization within a single framework offers a considerable edge in the market.
Another factor that distinguishes Syngene is its global reach and local compliance. Operating within Good Manufacturing Practice (GMP)-certified facilities, Syngene meets the regulatory standards of major markets such as the US, Europe, and Japan, ensuring that its testing protocols align with regional guidelines. This is particularly crucial for pharmaceutical companies with international supply chains that must adhere to varied regulatory expectations.
Collaborative Approach
Syngene’s collaborative approach also sets it apart from other contract organizations. Understanding that each client’s needs are unique, Syngene works closely with pharmaceutical companies to develop tailored testing protocols and risk assessment strategies. This partnership model allows for a more agile response to evolving regulatory requirements and helps companies stay ahead of the compliance curve.
Moreover, Syngene’s proactive stance in addressing future challenges in nitrosamine testing is evident in its research initiatives. As the pharmaceutical industry anticipates stricter regulations and the emergence of new nitrosamine contaminants, Syngene is already investing in the development of next-generation testing methodologies that will be crucial for maintaining drug safety in the years to come.

Conclusion
In an era where pharmaceutical safety and regulatory compliance are of paramount importance, the role of comprehensive nitrosamine testing cannot be overstated. Syngene International has positioned itself as a leader in this space, offering not only cutting-edge analytical services but also a holistic approach that integrates risk assessment, impurity profiling, and regulatory support. Its end-to-end capabilities provide pharmaceutical companies with the assurance they need to navigate the complexities of nitrosamine contamination while maintaining the highest standards of patient safety.
As the industry continues to grapple with the real-world implications of nitrosamine impurities, Syngene’s robust and agile solutions stand as a beacon for excellence, differentiation, and reliability in the global pharmaceutical landscape.
References:
- Syngene International - Analytical Development Services. https://www.syngeneintl.com/solutions/development/analytical-development/
- Syngene International - Nitrosamine Impurity Testing. https://www.syngeneintl.com/services/development/analytical-development/nitrosamine-impurity-testing/
- Syngene International Blog - Risk Assessment Strategy for Nitrosamine Impurities. https://www.syngeneintl.com/resources/blog/risk-assessment-strategy-for-nitrosamine-drug-substance-related-impurities/
- U.S. Food & Drug Administration (FDA) - Recalls and Safety Alerts. Information about Nitrosamine Impurities in Medications | FDA
- European Medicines Agency (EMA) - Reports on Nitrosamine Contamination in Drug Products. Nitrosamine impurities | European Medicines Agency (EMA) (europa.eu)
- Critical Analysis of Drug Product Recalls due to Nitrosamine Impurities | Journal of Medicinal Chemistry (acs.org)