Veeva Pulse Field Insights: Europe
Key Findings in Europe
Digital content boosts promotional response. U.S. data show a global trend that digital content used during video and in-person meetings more than doubles the promotional response over meetings that don’t share content.
Successful teams use content seven times more. Field reps that use digital content the most outpace seven times others who don’t frequently leverage it. European field teams have room for growth, currently sharing digital content in just 29% of meetings, despite its proven effectiveness.
High-impact content drives HCP engagement. In the last year, biopharmas created significantly more content. Yet, of all the content created, 76% is rarely or never used in Europe. This indicates a need for companies to focus their content strategy on developing fewer assets that are proven effective in advancing relevant engagements with HCPs.
Digital Content More Than Twice as Effective in Driving Promotional Response
Promotional impact of digital content
In-person and video meetings
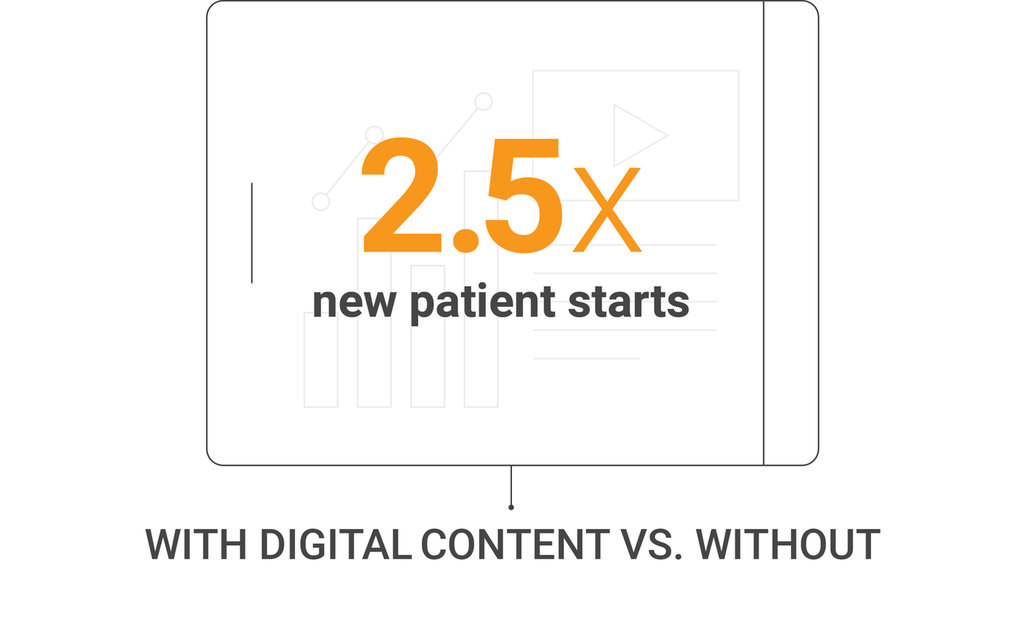
Veeva Pulse and Compass data, U.S.,
January 2019–June 2021
Percent of rep-HCP meetings using digital content
In-person and video
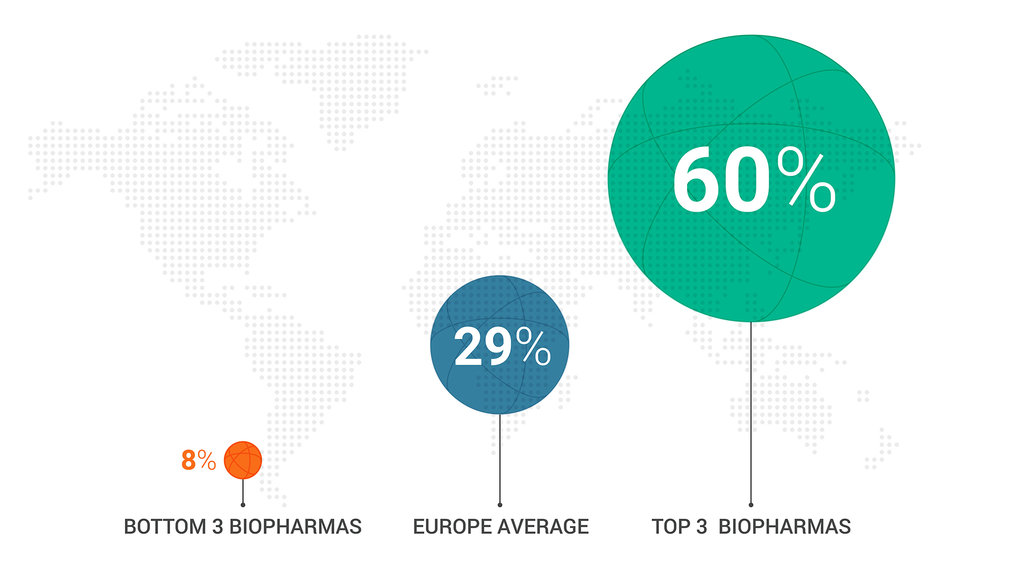
Veeva Pulse data,
April 2022–February 2023
HCP Engagement Metrics — Europe
Engagement Channel Mix Over Time
Veeva Pulse data, January–December 2022
HCP Video Meeting Duration

Veeva Pulse data, October–December 2022
FULL REPORT