Company Insight
Sponsored by Girteka
Girteka: Where Precision Meets Responsibility
How responsible logistics ensure the seamless delivery of pharmaceutical goods while meeting strict quality and environmental standards.
Main image credit:
Pharmaceutical logistics is a delicate thread in the European supply chain connecting vital medical products with the people who depend on them.
Sensitive cargo like medicaments and healthcare goods demands meticulous care—strict temperature control, secure handling, and compliance with regulations like Good Distribution Practices (GDP). Even a small disruption can compromise the integrity of this mechanism, making precision non-negotiable.
Yet, precision alone is no longer enough. As sustainability takes central stage in the European logistics market, the challenge lies in meeting existing quality standards while minimizing environmental impact.
Girteka, with nearly three decades of expertise in cold-chain logistics, seamlessly balances precision and sustainability, while ensuring safe transportation of sensitive and high-value cargo.
Smart Solutions for Complex Logistics
Operating across the European continent, Girteka is equipped to move healthcare products via road and rail, particularly when it comes to temperature-sensitive goods and high-security requirements. Standards like GDP, ISO 9001, and ISO 14001 shape the company’s day-to-day operations, while integrated digital and physical security measures like Real-Time Visibility (RTV) and TAPA TSR Level 1 compliant locks further protect the cargo integrity.
This adaptable infrastructure also allows for sustainable transportation options and a holistic approach tailored to various routes and needs:
- Electric Trucks: A zero-emission solution for short-haul trips (<450 km).
- Intermodal Rail Transport: A greener solution for long-distance freight movement (>800 km).
- Alternative Fuel Program (HVO 100): Supporting mixed-range deliveries, this sustainable fuel alternative aligns with environmental targets.
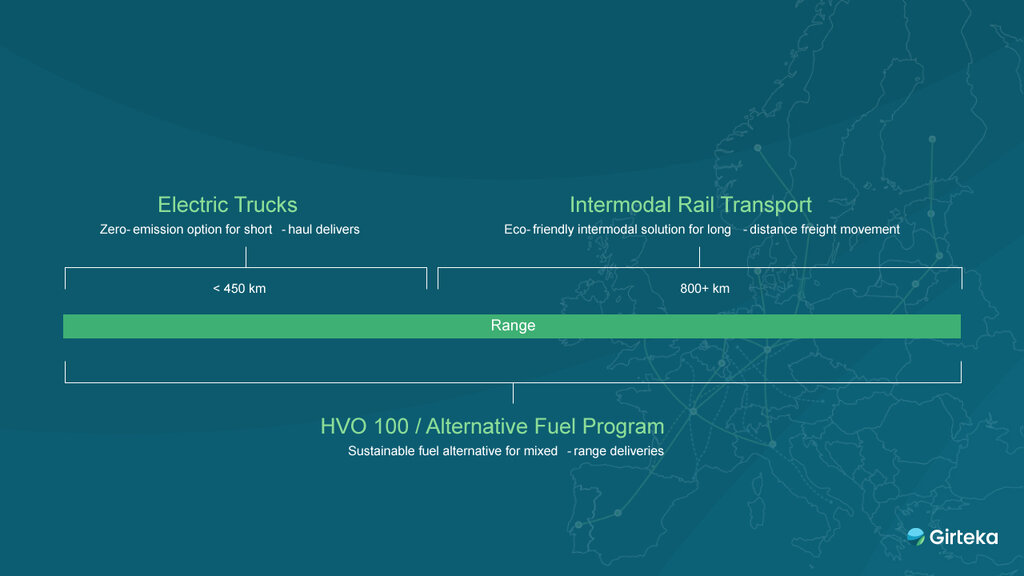
Girteka’s holistic approach helps to significantly reduce carbon footprint by combining various sustainable solutions for maximum impact.
Electric Vehicles in Action: A Case Study
In 2024, a collaboration with a German pharmaceutical partner allowed Girteka to plan, implement and test the effectiveness of Electric Vehicles (EVs) in reducing emissions within pharmaceutical logistics.
The project aimed to assess carbon emissions reductions compared to traditional diesel trucks by testing a full truckload (FTL) Battery Electric Vehicle (BEV) on a 300-kilometer domestic round-trip.
Initially, a theoretical simulation was prepared to evaluate the BEV’s impact on the chosen lane to compare its CO2 emissions with those of a standard diesel truck. The results during the project’s theoretical evaluation were promising, showing a 25% reduction in emissions when using electricity from the local grid:
- CO2 emissions using diesel truck: 408.3 kg per workday
- CO2 emissions using BEV (local grid): 300.7 kg per workday
- CO2 emissions using BEV (renewable energy): 0 kg per workday
Overcoming Challenges with Innovation
During the planning phase, several logistical challenges arose, particularly around on-site charging. Limited grid capacity at the client’s facilities meant that charging directly at both the departure and destination points was not feasible. To address this, the team collaborated with the partner to explore public charging alternatives nearby. They identified a charging station at an Autohaus close to the depot area; however, using this station required the vehicle to decouple for charging.
In the later phase, due to Girteka’s long-lasting partnership with one of the Europe largest trucks manufacturers, the team was able to secure the Mercedes-Benz eActros 600 electric truck with a 621 kWh battery capacity to test the prepared theoretical plan in practice. The chosen BEV optimized the route and eliminated the need for chargers and mid-journey decoupling, allowing for consistent, zero-emission travel when using solar-powered charging.
Over five days in September, the Mercedes-Benz BEV underwent rigorous testing along the 300-kilometer route. Key outcomes from this testing phase included:
- Reduced Stops: With only a single charging location needed mid-route, the team minimized the need for decoupling at the nearby warehouse charging spot.
- Enhanced Efficiency: The setup saved significant time by avoiding extra stops, improving both driving and loading efficiency.
- Energy Performance: The truck achieved a range of up to 550 kilometers on a single charge, outperforming initial expectations with lower-than-anticipated energy consumption.
- Environmental Impact: Using solar-powered public chargers enabled a consistent 100% CO2 reduction, demonstrating the viability of BEVs in pharmaceutical logistics under stringent environmental goals.
Following the project’s success, the partner is considering on-site charging to improve efficiency.
Co-Creating a Sustainable Future
In pharmaceutical logistics, sustainability is an ongoing journey built on collaboration and innovation. Recognizing that there’s no one-size-fits-all solution, Girteka works closely with its partners to develop tailored strategies that balance environmental objectives with operational needs.
This co-creation process supports the transition from conventional trucking to a new era of zero-emission transport. By embracing flexible and responsible solutions, these partnerships drive a greener, more sustainable future for pharmaceutical logistics.
“Today's world addresses many challenges. The one significantly affecting the logistics area is decarbonization. We are currently at a crossroads exploring potential green solutions while acknowledging the issues that lie ahead. In electrical transportation we need to work on these issues already today. This path cannot be walked alone, and open dialogue is fundamental in this collaboration. Only with joint efforts of various sectors, can we initiate a meaningful change and achieve remarkable results,” Viktorija Terekė, Head of Sustainability at Girteka.
