CONTACT US
Fully integrated CDMO offering tailor-made solutions
We provide tailor-made solutions to our customers by positioning ourselves as a fully integrated CDMO offering a wide range of premium services.
Our experienced team of specialists works to optimize target product profile and drug development program from early formulation development, scale-up, manufacturing and packaging to regulatory support.
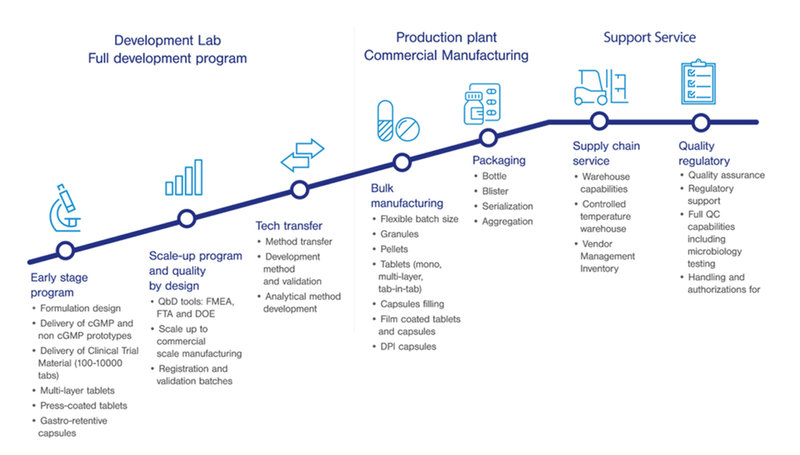

CDMO with a true D for Development:
Formulation development
We have extensive experience in solving complex formulation challenges. Our experts will advise you on the best solution to optimise drug delivery for the benefit to the patients. We could include our range of proprietary technologies to provide modified, pulse or timed release profiles to solve technological challenges, to provide patient benefits and to offer additional proprietary protection to the product.
Prototyping
We perform rapid and flexible prototyping essays to early characterized powders leading to anticipate potential up scaling issues which lead to save time and money.
Our key differentiators regards our compression simulator (Styl’One Evolution) and low scale equipment which reproduce industrial processes such as wet granulation, compression including tabletting and roller compaction and coating.
Product manufacturing for clinical trial
Once formulation is validated, we use our cGMP facilities to supply product for clinical study. Regulatory support is provided to assist you during clinical phases.
Controlled release technologies
Oral Drug Delivery Technologies
Skyepharma controlled release expertise is the result of years as a leader in drug development and oral drug delivery technologies serving the global pharmaceutical industry. Since it was founded, Skyepharma has had a strong focus on oral drug delivery, solving the many challenges of delivering the right dose of a drug to the right part of the gastrointestinal system for absorption at the right time and at the appropriate rate to achieve the desired therapeutic effect.
Oral Patented Technologies
3 patented pharmaceutical technologies: Geomatrix®, Geoclock®,and Soctec®. These platforms allow us to build controlled release formulations to adapt to patient dosing schedules or adjust API intake. These proprietary technologies allow us to better manage side effects
Brand new packaging lines - Serialization & aggregation:
Since 2018, oral dosage CDMO Skyepharma radically updated and expanded its packaging capabilities, opening new packaging lines equipped for serialization and aggregation.
WE ARE AVAILABLE FOR YOU
CONTACT US