Company Insight
Sponsored by Syngene
BIOSECURE Act 2025 update: what it means for biopharma outsourcing and what to do now
Click to edit...
Main image: Biosecure Act 2025 and its implications for biopharma supply chain risk and regulatory compliance. Generated by an LLM based on given prompts
The BIOSECURE Act is now law, folded into the FY26 Senate NDAA on December 18, 2025. The Biosecure Act will phase in government-wide restrictions on the use of “biotechnology equipment or services’’ from designated biotechnology companies of concern, affecting federal contracts, grants, and loans, and creating new biopharma supply chain risk.
Primarily, the BIOSECURE Act introduces two core restrictions for US executive agencies. First, agencies must not procure or obtain biotechnology equipment or services from a designated biotechnology company of concern. Second, agencies must not enter, extend, or renew a federal contract with an entity that uses covered biotechnology equipment or services in performing the federal contract. The enacted language also extends restrictions to certain federal loan and grant funds used to procure covered items or to contract in a way that would require the use of those items.
A key point is timing. The restrictions will roll out in stages. First, the government is expected to publish a list of designated companies, then issue guidance and update federal procurement rules. The restrictions will start after set lead times. Some existing contracts may be allowed to continue for a limited period, but they will still face transition deadlines later.
What to expect in 2026: biopharma supply chain risk outlook
While the contracting prohibitions don’t take effect immediately, the rollout is structured and time-bound. Think of it as a calendar that starts ticking the day the law is enacted. By December 18, 2026, the Office of Management and Budget is expected to publish the initial list of companies of concern (it can come earlier, but that is the deadline). After that list is out, OMB has 180 days to issue implementation guidance, so if the list lands close to the deadline, guidance would be due by June 16, 2027. Then the federal procurement rules have to catch up, with FAR revisions due within one year after OMB guidance, which puts the outside date at June 16, 2028 if guidance is issued at the last permissible moment.
The contracting prohibitions generally start 60 to 90 days after the FAR update, and some existing contracts may continue for a limited period under grandfathering or wind-down provisions. Certain Section 1260H entities can face faster application timelines, and the exact mechanics will be clarified through upcoming rulemaking, so the practical message is to track these dates and be ready to act as the list and rules become final.
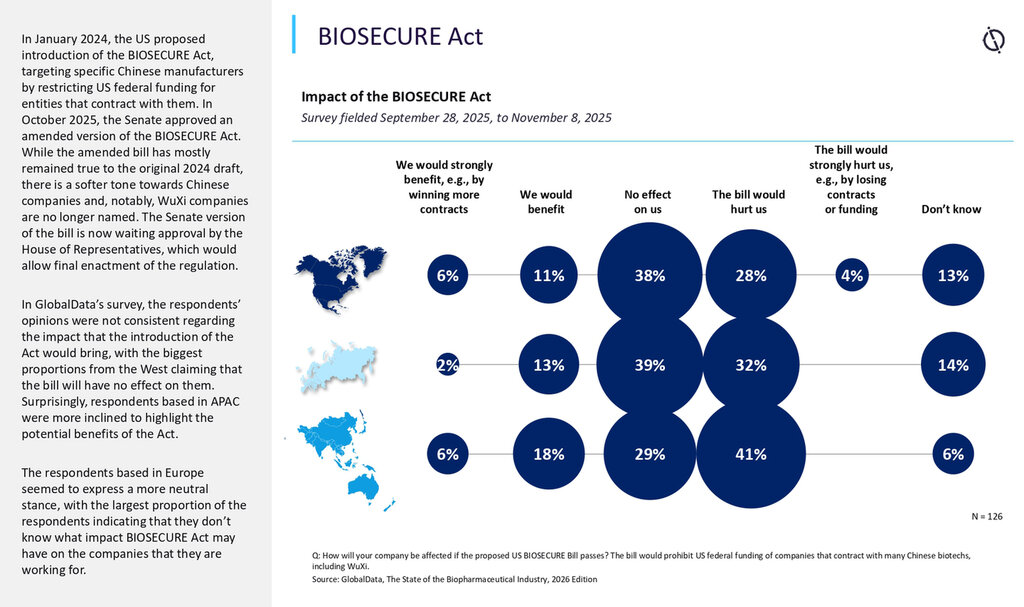
How this will potentially change the way CRO and CDMO outsourcing happens today
The Act does not only influence where work is performed. It also changes how work is performed across distributed CRO and CDMO networks. If a designated vendor touches an assay platform, critical consumables, data systems, or subcontracted testing, a program may need method transfers, bridging studies, tightened change control, and documentation updates to protect both eligibility and timelines. In practical terms, this is not a procurement issue alone. It becomes a technical and quality execution issue.
One reason the bar is rising is that designation mechanics are dynamic. The list of companies of concern will be compiled by the Office of Management and Budget through an interagency process and updated at least annually, with automatic linkages to the Department of Defense Section 1260H list. Companies can also seek removal through a formal process. This means exposure can change over time, and today’s acceptable workflow can become tomorrow’s constraint.
The second reason is that restrictions extend across funding pathways. Agencies are barred from procuring covered biotechnology items and from entering, extending, or renewing contracts if covered items are used in performance, and similar limits apply to federal grants and loans. The implication is that risk is not limited to one sponsor type or one contract type. It can follow the money, and it can reach deep into outsourced networks.
The immediate implications for your network
Subcontractor visibility becomes nonnegotiable
Sponsors will expect clear disclosure of who performs release testing, specialized assays, stability, and raw material qualification, as well as where raw data and audit trails live. When comparability evidence is spread across multiple labs and systems, investigations slow down, documentation gaps appear, and transitions become harder to defend.
Change control must link directly to supply decisions
Shifts in consumables, instrument platforms, or software are not minor administrative updates when eligibility is at stake. They require early notification, a clear impact assessment, and an agreed plan for bridging or requalification. Without this discipline, even routine supplier changes can trigger reactive work that disrupts timelines.
Technology transfer requalification is likely to become the bottleneck
Under constrained vendor options, moving work to a receiving site requires trained operators, comparable materials, validated methods, engineering runs, and qualification batches. For complex modalities, this can expand further into broader analytical bridging and stronger comparability justification. When these steps are not planned early, the delay usually shows up late, exactly when programs can least afford it.
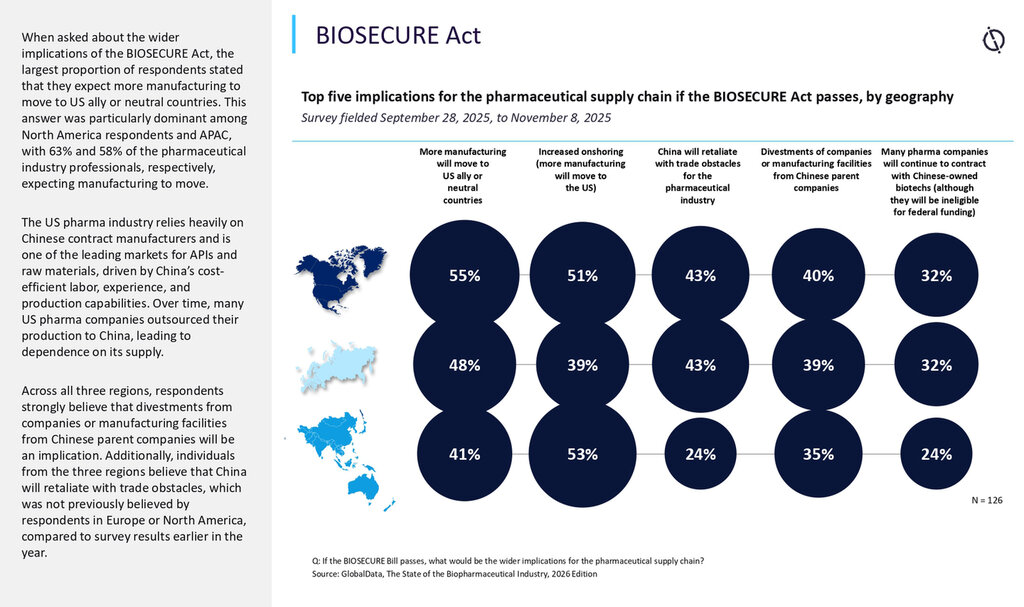
How Syngene is preparing to help their customers, partners and vendors
Building network resiliency for constrained vendor options
The BIOSECURE Act 2025 update makes it important to plan for constraints before they become urgent. A practical response is network resiliency: risk-tiered second-sourcing where feasible, and prequalified backup testing pathways so that critical work does not depend on a single vendor or system. When transitions are needed, governance matters as much as capacity. Clear change control triggers, early impact assessment, and well-defined bridging or requalification plans help keep timelines and quality stable.
Launching the mAb Accelerator Program to keep your pipeline moving
For many monoclonal antibody programs, the longest early step is cell line development, and it often gets delayed while sponsors complete internal approvals and funding. Syngene positions the mAb Accelerator Program to avoid that pause by starting cell line development immediately, with the stated goal of saving up to four months while those approvals are finalized. The intent is to build a stronger technical foundation early, so the program can move forward with fewer timeline shocks later.
That head start connects directly to the BIOSECURE Act 2025 update because time becomes a practical risk-control tool. Starting cell line development early creates room to map supplier dependencies, confirm subcontractor visibility, and align documentation and change control expectations in parallel, rather than rushing these steps after development has already been tied to specific tools, vendors, or data systems. If a vendor relationship later needs to change due to restrictions, programs with mature documentation, a clear method history, and tighter governance are typically in a better position to transition without losing control of records or rebuilding the comparability story from scratch.
To learn more and register for the mAb accelerator program, click here: mAb Accelerator Program - Syngene International Ltd
Learn more and register for the mAb Accelerator Program
SynVent and Hit‑to‑Lead: Programs That Keep Your Pipeline Moving
Early‑stage programs often lose momentum between scientific promise and operational follow‑through as sponsors navigate approvals, funding milestones, and evolving regulatory and supply‑chain demands. Syngene’s integrated discovery offerings SynVent and Hit‑to‑Lead, are designed to prevent those pauses by enabling programs to progress immediately within a single, coordinated framework.
SynVent integrates target triage, biology, medicinal chemistry, DMPK, and early safety under one program plan, allowing continuous data generation and earlier, better‑informed decisions. In parallel, Hit-to-Lead delivers rapid design–make–test–analyze cycles, SAR expansion, and early developability screening to mature hits into viable lead series.
In the context of the BIOSECURE Act, early integration becomes a risk‑management advantage, enabling standardized methods, clean data lineage, and vendor flexibility before assets are locked in. Together, SynVent and Hit-to-Lead help teams de‑risk pipelines, reach go/no‑go decisions sooner, and advance assets toward IND‑enabling studies with greater confidence.
Learn more about our integrated SynVent Platform
A practical way forward under Federal Acquisition Regulation (FAR) Changes and what to do now
Start with visibility that leads to action
Basic supplier maps are useful, but they do not show how quickly a critical activity can move if a vendor becomes constrained. A stronger approach is to build transfer-ready packages for the most important methods and processes, then assess each work package for single-sourcing risk, regulatory impact, method maturity, and estimated time to requalify.
Make data governance consistent across partners
During a transition, the limiting factor is often not capacity; it is whether raw data, metadata, and audit trails are complete and easy to retrieve across labs. Standardized naming, version control, and clear traceability to samples and methods can shorten investigations and speed up comparability packages when work needs to move.
Reduce reliance on single points of failure where it matters most
For sensitive inputs and high-impact assays, it helps to have alternate supply or testing paths identified early. Clear switch triggers, and pre-agreed bridging expectations such as system suitability and acceptance limits, can prevent rushed decisions later.
Keep contracts and governance aligned to the staged rollout
Vendor substitution provisions, subcontractor disclosure expectations, and change control requirements are easier to apply when they are built in up front. Regular governance reviews can also include monitoring of company designations and related updates that may affect the outsourcing network.
Summary
The BIOSECURE Act 2025 update is reshaping outsourcing by making supplier exposure and switching feasibility more consequential. Strong CDMO outsourcing compliance, early planning for technology transfer requalification, and realistic preparation for biotech vendor restrictions are becoming central to resilient execution. Treating biopharma supply chain risk as a measurable program parameter, rather than a static map, helps protect milestones while maintaining scientific rigor and regulatory confidence.
- Biffa internal savings data, average saving (January - August 2023)
- Nisbets internal savings data, average saving (January – September 2023)
- Compliance365 internal data, average savings
- HEINEKEN UK data, Calculations based on SmartDispense® active accounts each year since 2015 with 10 lines
- Independent test results based on subterranean and ground floor pub cellars of varying sizes
- Independent test results based on subterranean and ground floor pub cellars of varying sizes