Company Insight
Sponsored by DelSiTech
DelSiTech, innovative solutions in long-acting drug delivery
Biodegradable parenteral depot technology for long-acting and sustained release of small molecules, peptides and biologics.
Main image credit:
About us
DelSiTech is a clinical-stage drug delivery and development company and leading technology specialist in biodegradable silica-based controlled release of small molecule drugs, peptides, complex biologics, and vaccines.

The company works with its pharma and biotech partners to transform their active agents into novel and commercially viable therapeutic drug products.
DelSiTech™Silica formulated products can be dosed via different parenteral delivery routes such has subcutaneous, intramuscular, intratumoral, intrathecal, etc. DelSiTech silica matrix is also well fitted to ophthalmic products for topical administration (front of the eye). or intravitreal administration (back of the eye).
In addition, DelSITech develops its own controlled-release drug productsusing its proprietary technology platform.
Technology platform
DelSiTech™Silica technology platform is focusing on
- Long-acting injectable formulations for parenteral delivery from few days to several months - up to a year with a single injection
- Topical eye drops for ocular delivery for 24 to 48 hours with a single application.
How we differ
The patent-protected technology is based on biodegradable silica microparticles, and silica hydrogel depot has significant competitive advantages over other technologies, amongst them:
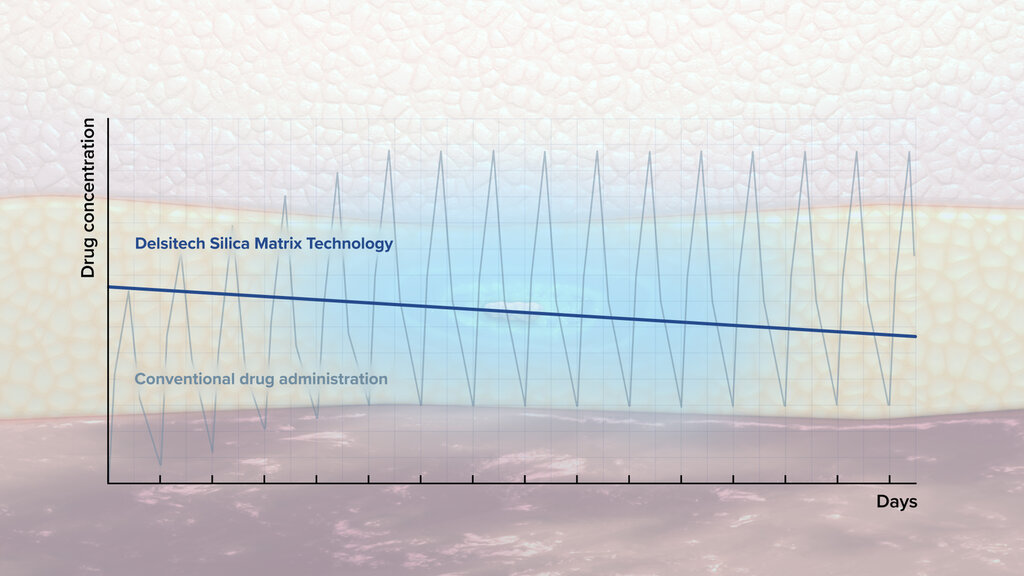
- Release: Typically, zero-order release kinetics
- Burst release: No burst release or low burst release
- Release duration: From few days to 1 year
- API solubility, size and nature: from almost non-soluble to highly water soluble active substances. Small molecules, peptides, to large (>1.000Kda) biological drugs and vaccines
- Increased stability: Active substances encapsulated in silica microparticles, are protected from degradation or external environment, keeping chemical and biological function intact
- Administration routes: Applicable for multiple different parenteral administration routes
- The only drug delivery technology applicable for both topical ophthalmic and intra ocular delivery
- Fast to develop as there is only few parameters to tune the release
The technology
DelSiTech™Silica is an advanced delivery technology for parenteral and local administration of injectable depot, implant and eye drop dosage forms. The proprietary technology is based on silica (SiO2) matrix into which the active ingredient is embedded using a process known as sol-gel. The resulting silica matrix can be designed to biodegrade by surface erosion at the required rate to ensure a tightly controlled release of the active substance over days to months, even up to a year.
Video animation on the technology.
Services
Our research and development service model is very flexible and accommodates the needs of small biotech to large pharmaceutical companies or non-profit organisations.
Formulation and Manufacturing
Typically, a new project starts with a feasibility study to demonstrate that silica technology is suitable for the active molecule. This includes development and manufacturing of preliminary silica-based formulations and testing of the drug release profile in in vitro dissolution studies according to the targets that have been set for the product. In addition, we can manufacture and supply, test products for preliminary animal studies to investigate the in vivo pharmacokinetics and/or efficacy of the controlled release product.
We offer spray drying and fill & finish manufacturing for GLP studies and early clinical trials either in our own clean room manufacturing unit or in our dedicated partner facilities under GMP. Technology transfer can also be made to our partner’s manufacturing site.

Analytical Services
With highly skilled and experienced scientists and state-of-the-art instrumentation, DelSiTech can deliver efficient and fast analytical services for product development. Our services include compound and formulation analyses, preliminary stability studies, degradation studies, dissolution testing and method development. We also offer tailor-made analysis of biological macromolecules such as peptides and proteins.
Partners
DelSiTech is working with a large number of companies in human and animal health. Our publicly announced partners are recognized global pharmaceutical companies as well as specialized biotechs (see more www.delsitech.com)
Our technology has been validated with several development and licensing alliances.
DelSiTech and Iveric Bio/ Astellas Exclusive Agreement for Development of Sustained Release Zimura®
DelSiTech and Tolmar Global License and Development Agreement
DelSiTech major licensing agreement on drug delivery
Contact information
DelSiTech Ltd
Itäinen Pitkäkatu 4C
FI-20520 Turku
Finland
Frederic Dargelas, PhD, MBA
Director, Head of Business Development and Alliance Management
Email: frederic.dargelas@delsitech.com
Web: www.delsitech.com