company insight
Specialty Chemicals for Pharmaceutical Industry
MOEHS is an European dynamic group of companies active in the pharmaceutical ingredients business. As a member of PMC Global, Inc., a California-based group, Moehs group has been established to coordinate the various activities of the companies of the group, with Moehs Ibérica, S.L. as the holding company.
Achieving compliance with local requirements can be a tough deal and an unexpected challenge sometimes
The main activity
API Manufacturer & Custom Manufacturer
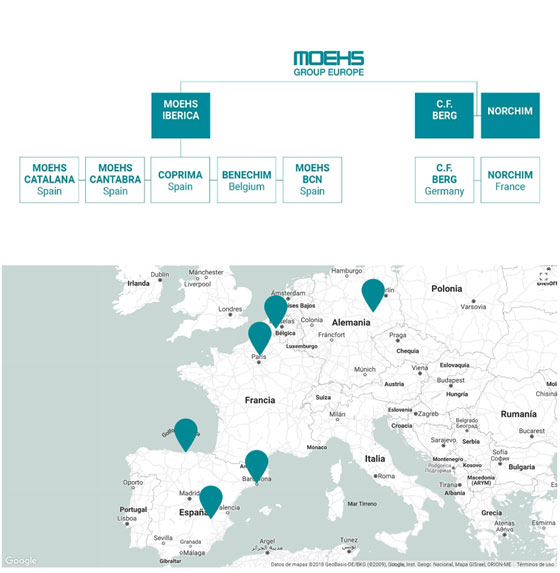
The main activity of the Group, since 1962, is manufacturing APIs & Custom Manufacturing for the pharmaceutical industry. The group has seven manufacturing sites highly flexible due to being multi-purpose plants:
Spain
- Moehs Catalana,
- S.L., Moehs Cántabra,
- S.L., Coprima, S.L.
- Moehs BCN, S.L.,
Belgium
- Benechim,
Germany
- Chemische Fabrik Berg
France
- Norchim

And our biotechnological brand Proteos Biotech, S.L. located in Spain and who develops and produces enzymes and proteins for biomedical and cosmetic applications.
MOEHS facilities
Along with R+D department and their background in the synthesis of complex organic compounds allow them to offer their customers a wide range of synthetic processes applicable industrial-wise that becoming MOEHS group in a standard in the manufacturing of Pharmaceutical actives for the generic market and one of the leaders in the Custom Manufacturing business. The success in Custom Manufacturing market segment depends on the confidence that the customer has for the industrial and technical response that company may provide.
Recently, MOEHS group built a new Research Center to expand its capabilities for R&D. The new Moehs Group Research Center is located in a 2.762 sqm building close to the manufacturing site of Moehs Catalana, S.L. The ground floor (1.800 sqm) host the laboratories for the synthesis and analysis development. The laboratories contain dedicated areas for liquid and gas chromatography, wet chemistry, solid state, high performance instrumentation, lab synthesis, kilo-lab (reactors ranging from 1L to 30L) and high-pressure reactions (reactors ranging from 100mL to 2L and up to 60Bar), leaving free space for future expansion. A contained area for HAPI development is projected to be constructed in that area in 2018. The second floor is devoted to offices for the management and the Regulatory Affairs, Toxicology and IP Departments in an aim to further integrate all the development teams, leaving a free space of 450 sqm for future expansion. The Research Center started activities in early September 2017.
Recently, MOEHS group built a new Research Center to expand its capabilities for R&D. The new Moehs Group Research Center is located in a 2.762 sqm building close to the manufacturing site of Moehs Catalana, S.L. The ground floor (1.800 sqm) host the laboratories for the synthesis and analysis development. The laboratories contain dedicated areas for liquid and gas chromatography, wet chemistry, solid state, high performance instrumentation, lab synthesis, kilo-lab (reactors ranging from 1L to 30L) and high-pressure reactions (reactors ranging from 100mL to 2L and up to 60Bar), leaving free space for future expansion. A contained area for HAPI development is projected to be constructed in that area in 2018. The second floor is devoted to offices for the management and the Regulatory Affairs, Toxicology and IP Departments in an aim to further integrate all the development teams, leaving a free space of 450 sqm for future expansion. The Research Center started activities in early September 2017.

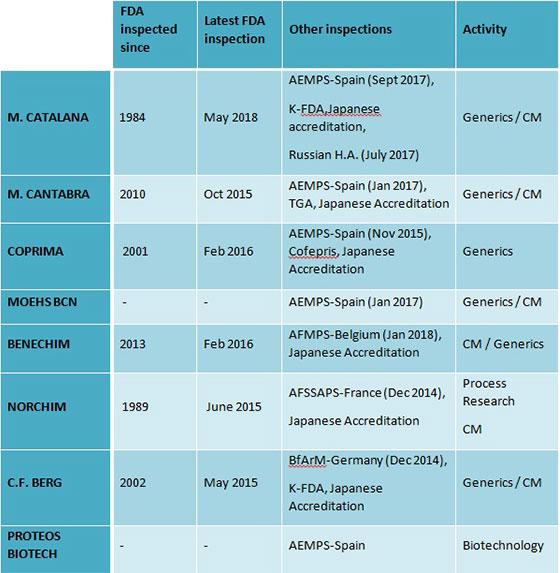
MOEHS Group produces and sells more than 200 products worldwide in more than 90 countries supported by the MOEHS Quality policy based in the cGMP international for Active Pharmaceutical Ingredients (API) manufacturers. These are CFR 210-211 and ICHQ7 guide. The different MOEHS factilities are regularly inspected by the local authorities and the FDA. In addition, their manufacturing plants are accredited by the PMDA, and some inspected by KFDA and TGA.
MOEHS combine the cGMP guides together with the input of their customers into a sole Quality Organization that considers all needs to have an efficient and continuous improvement of MOEHS service. Furthermore, MOEHS facilities are regularly inspected by their clients and have been routinely inspected by different authorities.
- ICH Q7 compliance
- Compliance to USP, EP, JP,ND
- More than 30 CEPs Issued
- 29 USDMF filed at the FDA
Also, MOEHS group has more than 80 DMFs of different APIs registered in more than 30 countries (including USA, Europe, Russia, Canada , Japan, Africa , Australia, New Zealand, and others). 29 USDMFs filed at the FDA for the US market and 16 J-DMF filed in the PMDA for the Japanese market and the European Department for the Quality of Medicines of the European Council has granted Certificates of Suitability (CEP or CoS) for 30 of MOEHS products and there are 3 dossiers which are currently under assessment. Moreover, MOEHS regularly collaborates with the European Pharmacopoeia and USP for the elaboration of new and revised monographs for several products and besides supplies to the EDQM and USP with reference standards of impurities and drug substances (CRS).
As you can see, with the solid basis of experience in the manufacturing of product with high levels of quality and safety standards-GMP’s, ICH, FDA and TGA approved, MOEHS group offers a large tradition and experience in the manufacturing of active pharmaceutical and veterinary ingredients, nutritional, cosmetic, as well as fine chemistry in general.