ImmuMap Services is a fast-growing Danish contract-research organization (CRO) with a global reach specialized in the development of customized flow cytometry assays for immune monitoring and immunological consulting.
Your lab in our hands
We are champions in assay development and use our scientific expertise to support our clients through all phases of drug development. As treatments and development projects varies greatly, our team of PhD-level scientists have a strong focus on developing assays to meet our customer’s needs for data-driven decision-making.
We strive for best-in-class solutions for our clients and aim to be an extension of your core facility. We provide personal expert consulting and services utilizing multicolor flow cytometry, ELISpot/fluorospot assays, cell culturing and a few high-end unique assays to assess immune status in patients and elucidate the effects of your immunotherapies in vitro and ex vivo. Our scientists work in close collaboration with our co-founder professor and key opinion leader Sine Reker Hadrup, PhD.
learn more
Scroll down

The ImmuMap way
a strong focus on assay development to meet customer needs

Our primary focus is to develop cutting-edge assays for immune monitoring with a strong commitment to customization, optimization and validation of our solutions living up to our rigorous quality control standards to ensure reliable results. We offer several front-line solutions for determining immune reactivity and immunogenicity.
We tailor standard assay formats such as the following for answering your specific research question:
- Immunophenotyping for differentiation and maturation markers
- Intracellular cytokine staining for cytokine profiling and functionality
- MHC multimer staining for precise detection of antigen-specific T cells
- Single-analyte ELISpot and multi-analyte fluorospot

Our services are used in many therapeutic areas and have already been applied in immune oncology, autoimmunity and safety assessment of cell products. We support our customers in all phases of drug development, from QC, discovery and pre-clinical to clinical testing in phase I, II and III. In addition, we have a trusted provider of acute reaction whole-blood loop assay analyses.
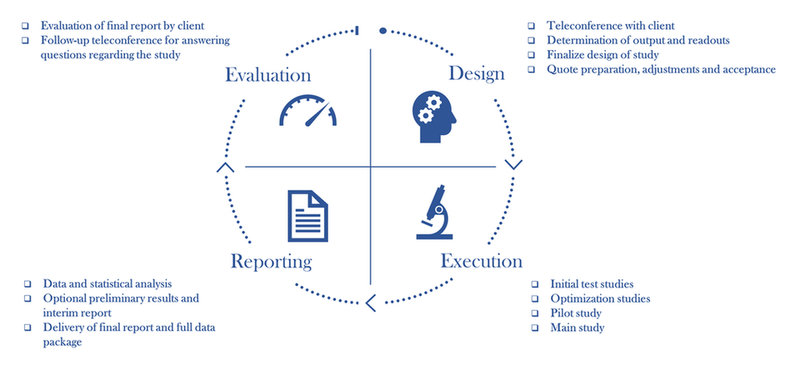
We are agile and flexible and thus have no constraints in fulfilling our clients’ requests whether regarding measurement of rather “exotic” markers or living up to tremendously tight deadlines for data delivery. We provide an end-to-end study solution including design, wet lab execution, reporting and evaluation. For all steps, we closely collaborate with our clients to ensure that the analyses performed meet the individual client’s objectives for the study. The final product is a detailed report with statistical analyses and interpretation of the results alongside access to the complete data package.
HUMAN IFN-γ ELISPOT ASSAY PRECISION
We have tested the assay precision including repeatability (intra-assay variability) and reproducibility (inter-assay variability). Initial experiment (day 1) was performed on 5 donors against 2-5 cytomegalovirus (CMV), Epstein Barr virus (EBV) or influenza derived peptide epitopes each and tested in triplicates (data shown for 2 selected donors). Experiments on day 2 and 3 were performed on two selected donors against one HLA-matched CMV-derived peptide each and tested in six replicates.
Repeatability
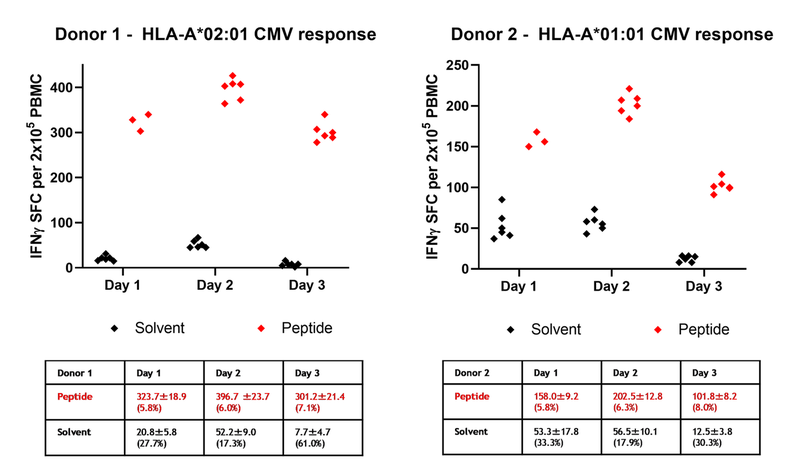
Figure 1. Results from analysis of two donors across three independent days. Scatter plot of IFNγ spot forming cells (SFC) per 2x105 PBMC from Donor 1 (left) and Donor 2 (right). Solvent spots (background) (black) in PBMC stimulated with medium inclusive peptide solvent (DMSO), peptide spots (red) in PBMC stimulated with an HLA-A*02:01-restricted CMV peptide (donor 1) or an HLA-A*01:01-restricted CMV peptide (donor 2). The intra-day mean, standard deviations and CV for donor replicates are shown in the tables.
Reproducibility
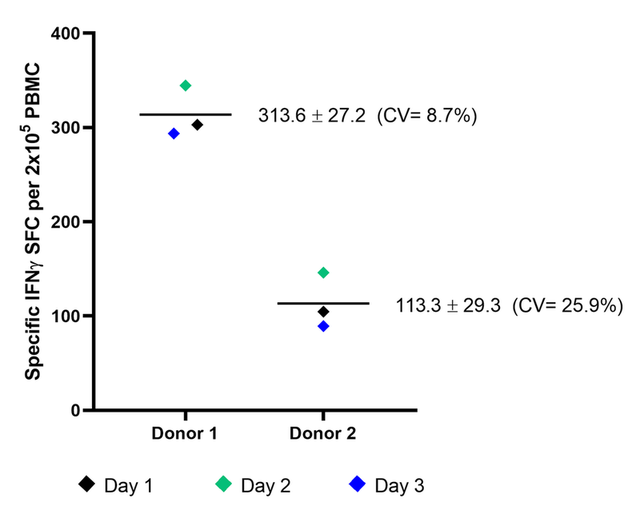
Figure 2. Mean of peptide-specific spots from two donors across three independent days. Scatter plot of the average number of IFNγ spot forming cells (SFC) per 2x105 PBMC from Donor 1 (left) and Donor 2 (right). Background spots are subtracted from peptide-stimulated spots to enumerate the number of peptide-specific spots. The average number of peptide specific spots for day 1 (black), day 2 (green) and day 3 (blue) as well as the mean value across all three days (black line) are depicted. Values for the inter-day mean, standard deviation and CV% for donor replicates are listed.
Our services for your next immune monitoring study
We enjoy the challenge of putting together complex immune monitoring studies and offer completely customer-tailored assays. We also offer a wide range of standardized assays for mapping cellular immunity and assessing immune status in patients or animals.
Assays provided by ImmuMap Services includes:
- Multicolor flow cytometry, up to 18 parameters simultaneously
- Intra-cellular multicolor flow cytometry, including cytokine and transcription factor profiling
- Detection of low frequency T cell populations by
- MHC-multimer staining
- Multiple parallel MHC-multimer stainings, including dCode DNA barcoded MHC multimers
- Fluorescence-activated cell sorting
- Elispot
- Fluorospot
Additional services provided by ImmuMap includes, but are not limited to:
- Whole blood processing for obtaining peripheral blood mononuclear cells (PBMCs), serum or plasma with following cryopreservation. Species: human, mouse and pig
- Cell culturing of primary cells, TCR products, cancer cell lines etc. for in vitro experiments. Species: human and mouse
- Cytotoxicity, activation and proliferation assays for assessing alloreactivity, cytotoxicity of compounds or the killing or proliferative capabilities of cellular products or sorted antigen-specific T cells. Species: human, mouse and pig
- Fully customer-tailored assays combining the above services in one immune monitoring study.
- Consultation regarding optimal immunologic assessment and assay development
- Training in the above methods
- Development/optimization of protocols for your in-house flow cytometry facility
Contact us to learn what we can do for you.
ImmuMap
Diplomvej 377
2800 Kongens Lyngby
Denmark

Go to top